��Ŀ����
ijʵ��С��ģ���¯�����Ļ�ѧ��Ӧԭ������ʵ�飬��װ������ͼ��ʾ��
��1��A��������Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�з�����Ӧ�Ļ�ѧ����ʽ����������������������
C����ȼ�ƾ��Ƶ�Ŀ����������������������������
��3��ʵ�������С�����ɫ����ȫ������ˣ���Ϊ������ǵ���������С����������ɣ����п��������������ʡ�Ϊ�˽�һ����֤�����ɷ֣�С���鵽�������ϣ����������������������������������������������û����������У�2����3���ֻ��ϼۣ�������������Ϊ����ɫ�����Ϊ��ɫ������ֻ�������������ܱ����������������������ﶼ�����ᷢ����Ӧ���ܽ⡣�ۻ�ɫ����Һ������Fe3+����Һ�����Ȼ���������������������
����������Ϣ��С�������������ʵ�顣
[ʵ��1]
����ɫ������ĥ��ȡ������������������Һ�У��۲쵽�����ݳ��֣���Һδ���ɫ���ݴ˵ó��Ľ�����������������������������
[ʵ��2]
���ô����������º�ɫ���壬ʵ��ɶԺ�ɫ����ɷֽ����жϣ�������������� ����� ��
��1��3CO��Fe2O3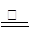 2Fe + 3CO2
2Fe + 3CO2
��2��CO2��Ca(OH)2�� CaCO3�� + H2O ��ֹCO��Ⱦ����
��3��ϡ���ᣨ��ϡ���ᣩ �е���������Fe3O4
�����ɫ���屻����ȫ��������˵��ֻ�е����������������������˵����ɫ����ΪFe��FeO��
�������������һ����̼��ԭ�������������Ͷ�����̼�������Ǻ�ɫ�Ĺ����ɺ�ɫ�����ɵĶ�����̼��ʹ�����ʯ��ˮ����ǣ�ʣ���β���к���һ����̼����ͨ��ȼ�շ���ȥ��
���ݡ��۲쵽�����ݳ��֣���Һδ���ɫ��˵�����е��������������������ٸ��ݡ�ֻ�������������ܱ��������������ô����������º�ɫ���壬ʵ��ɶԺ�ɫ����ɷֽ����жϣ�����ɫ���屻����ȫ��������˵��ֻ�е����������������������˵����ɫ����ΪFe��FeO��
���㣺ʵ��̽��

 ������ϵ�д�
������ϵ�д�
��1�����Ͻ������������г��ij��ܣ����������кܺõĿ���ʴ���ܣ��û�ѧ����ʽ������ԭ��
��
��2��Ϊ��ֹ���г����⣬���������к������� ������ĸ����
| A�����ܱ�����Ϳ���� | B����Ȧ����Ʒ������� |
| C����������Ϳ���� | D��¶����ã���ɹ���� |
���ڳ�ʪ�Ŀ�����ᷢ����ʴ��֤������һ���μ��˷�Ӧ����Ҫ����ʵ����( )
| A���٢� | B���٢� | C���ڢ� | D���٢ڢ� |





