��Ŀ����
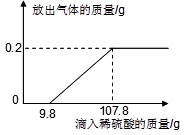
��8�֣��Ȼ�����һ����Ҫ�Ļ���ԭ�ϡ�����Ȼ�����Һ���Ƶ��������������Ƶ����ʣ���Ӧ�Ļ�ѧ����ʽΪ2NaCl + 2H2O 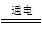 Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺
Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺
(1)�Ȼ�����ȫ��Ӧʱ����Һ��ʣ��ˮ��������
(2)ԭ�Ȼ�����Һ�����ʵ�����������
(3)������Щ�Ȼ�����Һϡ�ͳ����ʵ���������Ϊ0.9%��������ˮ����Ҫ��ˮ��������
 Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺
Cl2��+ H2��+ 2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ���ǡ����ȫ��Ӧʱ���õ�51.2 g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ������㣺
(1)�Ȼ�����ȫ��Ӧʱ����Һ��ʣ��ˮ��������
(2)ԭ�Ȼ�����Һ�����ʵ�����������
(3)������Щ�Ȼ�����Һϡ�ͳ����ʵ���������Ϊ0.9%��������ˮ����Ҫ��ˮ��������
(��8��)�⣺(1)�������Ȼ��Ƶ�����Ϊx�������������Ƶ�����Ϊy����������������Ϊz
2NaCl + 2H2O Cl2��+ H2��+ 2NaOH
Cl2��+ H2��+ 2NaOH
2��58.5 71 2 2��40
x 7.1 g z y
�÷��� (2��58.5)��71 =" x" ��7.1 g ��1�֣�
71��(2��40) =" 7.1" g ��y ��1�֣�
71��2 =" 7.1" g ��z ��1�֣�
��� x =" 11.7" g y =" 8" g z =" 0.2" g
��ʣ��ˮ��������51.2 g �C 8 g =" 43.2" g ��1�֣�
(2)�Ȼ�����Һ����Ϊ��51.2 g + 7.1 g + 0.2 g =" 58.5" g ��1�֣�
�Ȼ�����Һ�����ʵ���������Ϊ��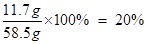
��1�֣�
(3)��ϡ�ͺ�������ˮ������Ϊa
58.5 g��20% = a��0.9%
2NaCl + 2H2O
 Cl2��+ H2��+ 2NaOH
Cl2��+ H2��+ 2NaOH2��58.5 71 2 2��40
x 7.1 g z y
�÷��� (2��58.5)��71 =" x" ��7.1 g ��1�֣�
71��(2��40) =" 7.1" g ��y ��1�֣�
71��2 =" 7.1" g ��z ��1�֣�
��� x =" 11.7" g y =" 8" g z =" 0.2" g
��ʣ��ˮ��������51.2 g �C 8 g =" 43.2" g ��1�֣�
(2)�Ȼ�����Һ����Ϊ��51.2 g + 7.1 g + 0.2 g =" 58.5" g ��1�֣�
�Ȼ�����Һ�����ʵ���������Ϊ��
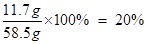
��1�֣�
(3)��ϡ�ͺ�������ˮ������Ϊa
58.5 g��20% = a��0.9%
��������ͼ�ɿ������Ȼ�����ȫ��Ӧʱ�ɵõ�����7.1g��
��1����Ӧ������������Һ�ڰ����������ƺ�ˮ����������������������������Ƶ����������÷�Ӧ������������Һ������-�������Ƶ�������Ϊ��Һ��ʣ��ˮ��������
��2��ԭ�Ȼ�����Һ�����ʵ���������= ��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ��������
��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ��������
��3������ϡ��ǰ����Һ�����ʵ����������г���ʽ��
��𣺽⣺��1���������Ȼ��Ƶ�����Ϊx�������������Ƶ�����Ϊy����������������Ϊz
2NaCl+2H2O Cl2��+H2��+2NaOH
Cl2��+H2��+2NaOH
117 71 2 80
x 7.1g z y
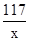 =
=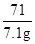
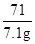 =
=
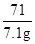 =
=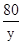
x=11.7g z=0.2g y=8g
ʣ��ˮ��������51.2g-8g=43.2g
����Һ��ʣ��ˮ������Ϊ43.2g
��2���Ȼ�����Һ����Ϊ��51.2g+7.1g+0.2g=58.5g
�Ȼ�����Һ�����ʵ���������Ϊ��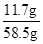 ��100%=20%
��100%=20%
��ԭ�Ȼ�����Һ�����ʵ���������Ϊ20%��
��3����ϡ�ͺ�������ˮ������Ϊa
58.5 g��20%=a��0.9%
a="1300" g
��Ҫ��ˮ������Ϊ��1300 g-58.5 g="1241.5" g
��������Щ�Ȼ�����Һϡ�ͳ�0.9%��������ˮ����Ҫ��ˮ������Ϊ1241.5 g��
������ϡ����Һ�Ĺ���ֻ�Ǽ�ˮ�Ĺ��̣�ϡ��ǰ����Һ�����ʵ��������䣬��������й�ϡ����Һ�ļ�����ʱ�ɸ���ϡ��ǰ����Һ�����ʵ����������г���ʽ��
��1����Ӧ������������Һ�ڰ����������ƺ�ˮ����������������������������Ƶ����������÷�Ӧ������������Һ������-�������Ƶ�������Ϊ��Һ��ʣ��ˮ��������
��2��ԭ�Ȼ�����Һ�����ʵ���������=
 ��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ��������
��100%���Ȼ��Ƶ������ɸ���������������������������غ㶨�ɣ��Ȼ�����Һ������=����������+�������������ɸ������������������+��Ӧ������������Һ����������3������ϡ��ǰ����Һ�����ʵ����������г���ʽ��
��𣺽⣺��1���������Ȼ��Ƶ�����Ϊx�������������Ƶ�����Ϊy����������������Ϊz
2NaCl+2H2O
 Cl2��+H2��+2NaOH
Cl2��+H2��+2NaOH117 71 2 80
x 7.1g z y
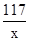 =
=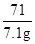
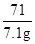 =
=
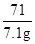 =
=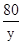
x=11.7g z=0.2g y=8g
ʣ��ˮ��������51.2g-8g=43.2g
����Һ��ʣ��ˮ������Ϊ43.2g
��2���Ȼ�����Һ����Ϊ��51.2g+7.1g+0.2g=58.5g
�Ȼ�����Һ�����ʵ���������Ϊ��
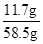 ��100%=20%
��100%=20%��ԭ�Ȼ�����Һ�����ʵ���������Ϊ20%��
��3����ϡ�ͺ�������ˮ������Ϊa
58.5 g��20%=a��0.9%
a="1300" g
��Ҫ��ˮ������Ϊ��1300 g-58.5 g="1241.5" g
��������Щ�Ȼ�����Һϡ�ͳ�0.9%��������ˮ����Ҫ��ˮ������Ϊ1241.5 g��
������ϡ����Һ�Ĺ���ֻ�Ǽ�ˮ�Ĺ��̣�ϡ��ǰ����Һ�����ʵ��������䣬��������й�ϡ����Һ�ļ�����ʱ�ɸ���ϡ��ǰ����Һ�����ʵ����������г���ʽ��

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
�����Ŀ
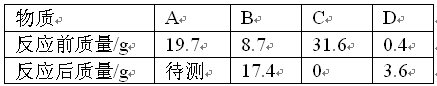

 Na2SO4 + H2O + CO2����
Na2SO4 + H2O + CO2���� 1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ��
1g/cm3����Ȼ�������صμ�7.4%�ij���ʯ��ˮ������ȥ100�˳���ʯ��ˮʱ��̼����ǡ�÷�Ӧ��ȫ�� ������ȷ��0.01����
������ȷ��0.01����