��Ŀ����
����Ŀ����ѧ����Ϊ������ͽ���Ϊδ������Ҫ��Դ����2017��10����������ʹ����ȼ�ϵ�ص��й�糵�ںӱ���ɽͶ����ҵ��ת����־���ҹ�����Դ����������һ����̨�ס�
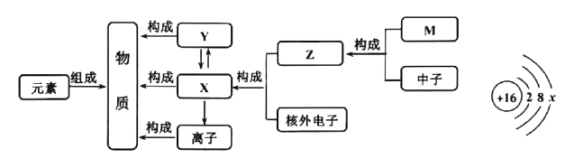
��1����ȼ�ϵ���ǽ�_____��ת��Ϊ���ܡ�
��2����������Ϊ����ɫ��Դ������Ҫԭ����_____��
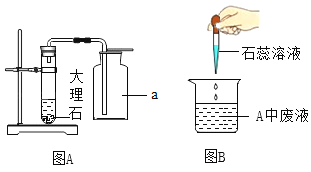
��3����ͼ��ʵ���еij���װ�ã���ش��������⣺
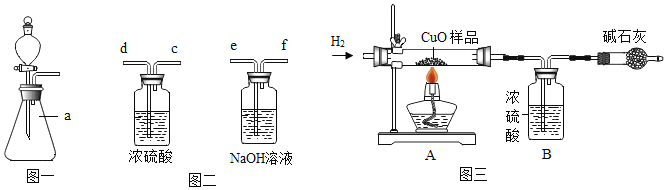
�������a������_____��
��ʵ���ҳ���ͼһװ������ȡ��������Ӧ�Ļ�ѧ����ʽΪ_____����װ�õ��ŵ���_____��
������ϡ������ȡ�������Ậ���������ʣ���ͼ��װ�ÿɵõ����������������������������ӽ�˳����ȷ����_____������ĸ����
��4���������������������������ͼ����ʾ��ʵ�飬�ⶨ��Ϲ�����CuO��������������֪����ӦǰAװ����CuO��Ʒ������Ϊm1g������Ʒ�е��������ʲ���H2��Ӧ������Ӧ������װ��B����m2g������m1��m2��ʾ��Ʒ��CuO����������Ϊ_____��
���𰸡���ѧ ������������ȼ��ֻ��ˮ���Ի�������Ⱦ ��ƿ Zn+H2SO4��ZnSO4+H2�� �ܹ����Ʒ�Ӧ������ efd ![]()
��������
��1����ȼ�ϵ���ǽ���ѧ��ת���ɵ��ܣ������ѧ
��2����������Ϊ����ɫ��Դ������Ҫԭ���ǣ�������������ȼ��ֻ��ˮ���Ի�������Ⱦ�����������������ȼ��ֻ��ˮ���Ի�������Ⱦ
��3��������a�����ƣ���ƿ�������ƿ
��п��ϡ���ᷴӦ��������п����������ѧ����ʽΪ��Zn+H2SO4��ZnSO4+H2������װ�õ��ŵ��ǣ��ܹ����Ʒ�Ӧ�����ʣ����Zn+H2SO4��ZnSO4+H2�����ܹ����Ʒ�Ӧ������
����Ϊ�������Ʋ���������Ӧ�ܺ��Ȼ������巴Ӧ��Ҫ��ͨ��ʢ����������Һ��װ����ͨ��װ��Ũ�����װ�ý��и�����Ե�����������ӽ�˳����ȷ���ǣ�efd�����efd
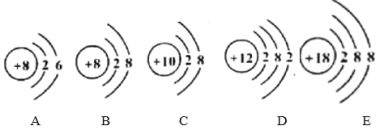
��3������ͭ����Ԫ�ص�����Ϊ��![]() ����Ʒ������ͭ������Ϊ��
����Ʒ������ͭ������Ϊ��![]() ����Ʒ������ͭ����������Ϊ��
����Ʒ������ͭ����������Ϊ��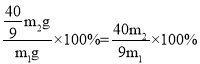 �����
�����![]()

 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�����Ŀ�����о�������кͷ�Ӧ��ʱ��ij��ȤС��ͬѧ��֤��ϡ����������������Һ��Ϻ��Ƿ����˻�ѧ��Ӧ������������̽����
��1����ʢ������������Һ���ձ����뼸�η�̪��Һ������μ���ϡ���ᣬ�����Ͻ��裨��ͼһ���������ֻ��������������������������Һ���ǰ����¶ȱ仯�������ͼ������
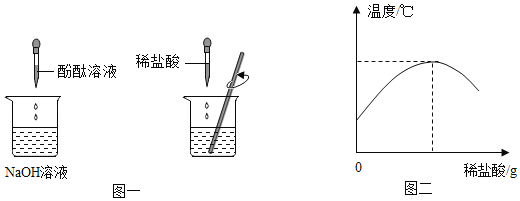
��ʵ����������߱仯���������֪�����������������Һ�����˷�Ӧ��_____������ա��ų�����������
��2�����۽Ƕȷ�������������������Һ�ķ�Ӧ
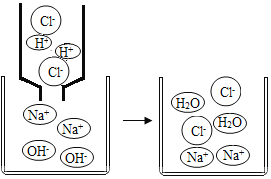
����ͼ��֪���÷�Ӧ����ʵ����_____��
���������ۣ�ͬѧ�����ۺ���Ϊ�����������������Ƽ��٣�����ʧ�������������������ɣ�����֤����Ӧ�Ѿ�������
��3��̽��ʵ����ձ������ʵijɷ֡�
���� | ���� | ���� |
ʵ��1��ȡ�ձ��е���Һ�������Թ��У���������ͭ��Һ | _____ | ֤��û��NaOH |
ʵ��2����ȡ�ո��е���Һ�������Թ��У�������������Һ | ���ְ�ɫ���� | ֤������HCl |
ʵ��3����ȡ�ձ��е���Һ�������������У��������� | ��_____���� | ֤����NaCl���� |
���ó����ۣ�����������Һ�����ᷢ�����кͷ�Ӧ��
�����۷�˼��
����ͬѧ��Ϊ����ͨ��ʵ��2������Һ�к���HCl��֤�ݲ��㣬������_____��
�������кͷ�Ӧ�����Խ�������������е�ʵ��ͬ�⡣�繤���ð�ˮ��NH3H2O��������ˮ�е����ᣬ�ܵõ�һ�ֵ���һ����泥�д���÷�Ӧ�Ļ�ѧ����ʽ_____��
��Ƕ���ʶ��ѧ��Ӧ��ѧϰ��ѧ���ر䷽����
����Ŀ������ͬѧͨ���Ķ��������ϵ�֪��DZˮͧ�г��ù������ƣ�Na2O2����Ϊ���������йط�Ӧ�Ļ�ѧ����ʽΪ��2Na2O2+2CO2�T2Na2CO3+O2��2Na2O2+2H2O�T4NaOH+O2��������������ͼ����װ������ȡCO2����֤����Na2O2�ķ�Ӧ��
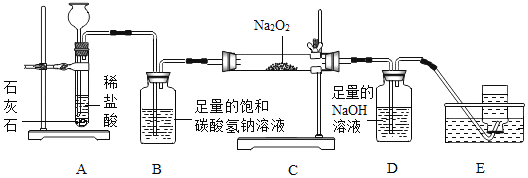
��1��װ��B��������_____��
��2����Ӧһ��ʱ���װ��E���ռ�����������Ҫ��_____����Ӧ��װ��CӲ�ʲ������й���ijɷ���ʲô������Ϊ���ֽ���������̽����
����������裩
����һ Na2CO3
�����Na2CO3��Na2O2
������Na2CO3��NaOH
������������_____
�����ʵ�飩
ʵ����� | ʵ������ | ʵ����� |
��ȡ������Ʒ���Թ��У�����������ˮ���� | ������ȫ�ܽ⣬_____ | ��Ʒ��һ��û��Na2O2 |
��ȡ����ʵ����������Һ����һ�Թ��У����������BaCl2��Һ���� | �а�ɫ�������� | ��������ȷ |
��ȡ����ʵ���������ϲ���Һ����һ�Թ��У�����_____��Һ���� | �а�ɫ�������� |
����˼�����ۣ���Ӧ��װ��CӲ�ʲ������еĹ��庬��NaOH��ԭ�������_____��
����Ŀ��ij��ѧ��ȤС���ͬѧ���տα��ϵ�ʵ��װ�������ⶨ�����������������������ʵ��(��ͼ��ʾ),������������������������С��1/5.
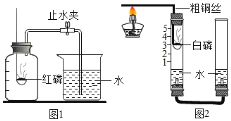
�������̽����
��������⣩�������ԭ����ʲô?��θĽ��α��ϵ�ʵ��װ��?
���������ϣ����ס�����һЩ�������±���
��ɫ��״̬ | �۵�/�� | �Ż��/�� | �ܶ�/(g/cm3) | |
���� | ����ɫ���� | 590 | 240 | 2.34 |
���� | ��ɫ���ɫ���� | 44.1 | 40 | 1.82 |
����������裩
��ͬѧ�������ǵ�����ԭ���п������������������ˮ��Ӱ��ʵ���ȷ�ԡ�
��ͬѧ��������ƿ�ڲ��������л���������
�㻹�������IJ�����____________.
�����������ۣ�
(1)��ͬѧ��Ϊ��ľ̿������ף��Ϳ��������������Ϊ���IJ��벻������������___.
(2)���ǶԿα��ϵ�ʵ��װ�ý�������ͼ��ʾ�ĸĽ�(������������).
�ټ�ͬѧ���Ӧ�Ѻ���Ϊ����,�����ȼ�յ�����_________.(�������ͬ)
����α��ϵ�ʵ��װ����Ƚϣ���װ�õ�һ���ŵ���____________.
��ͨ����ͭ˿����ȼ�������ڵİ��ף����������˽�����_________�ԡ�