��Ŀ����
�á����������Ƽ���ƵõĴ�������������Ȼ��ơ�Ϊ�ⶨij������Ʒ��̼���Ƶĺ�����С����ȡ�ô�����Ʒ12.4g���������μ������ʷ���Ϊ10����ϡ���ᡣ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺
��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH 7���������������
��2����������C�����ֵΪ g��
��3����ô�����Ʒ��̼���Ƶ�����������������������һλС����
��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH 7���������������
��2����������C�����ֵΪ g��
��3����ô�����Ʒ��̼���Ƶ�����������������������һλС����

��11�֣���1���� ��2�֣�
��2��4.4 ��2�֣�
��3���⣺�贿����Ʒ��̼���Ƶ�����ΪX
Na2CO3+2HCl="==" 2NaCl+H2O+CO2�� ��1�֣�
106 73
X 73g��10�� ��1�֣�
106:73=X: 73g��10��
X="7.3g " ��2�֣�
������Ʒ��̼���Ƶ���������Ϊ�� ��2�֣�
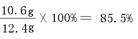
�𣺣��ԣ� ��1�֣�
��2��4.4 ��2�֣�
��3���⣺�贿����Ʒ��̼���Ƶ�����ΪX
Na2CO3+2HCl="==" 2NaCl+H2O+CO2�� ��1�֣�
106 73
X 73g��10�� ��1�֣�
106:73=X: 73g��10��
X="7.3g " ��2�֣�
������Ʒ��̼���Ƶ���������Ϊ�� ��2�֣�
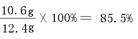
�𣺣��ԣ� ��1�֣�
��

��ϰ��ϵ�д�
�����Ŀ

