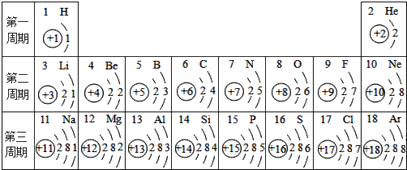
��Ŀ����
����Ŀ����Դ����Դ�뻷���ѳ�Ϊ���������ע�����⡣
���ϣ���ȼ�����������ڵ����ϵĴ�����Լ����̽��������ʯȼ��������2����ͬ�������£���ȼ��ȼ�ղ����������ȴ�ͳ�Ļ�ʯȼ��Ҫ�����ʮ��������ȼ�պ����κβ����ͷ������ǹ��ϵĵ�������δ�����Ĵ����ḻ��������Դ��
��1��Ŀǰ������ʹ�õĻ�ʯȼ����Ҫ����ú��ʯ�͡�_______��
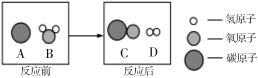
��2��Ϊ������Ⱦ�����ú�������ʣ����ڸ��������½���ת��Ϊ��ȼ�����壬�˹��̵���ʾ��ͼ���£��÷�Ӧ�Ļ�ѧ����ʽ��___________��
��3��Ϊ������������䣬��Ȼ��ͨ����ѹ�����ݻ���С�ĸ�ƿ�У�����ȼ��ȴ���ѱ�ѹ�����Դӷ��ӵĽǶȷ��������е�ԭ����_________________��
��4����ȼ����Ϊ������Դ����ȴ�ͳ��ʯȼ�Ͼ��кܶ��ŵ㣬��Ҫ��_____������ţ���
A �����ḻ B ȼ��ֵ�� C ��ࡢ����Ⱦ D ���ڿ���
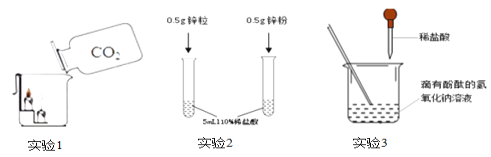
��5���ø��������һ����̼��ԭ��������ʵ��װ����ͼ��
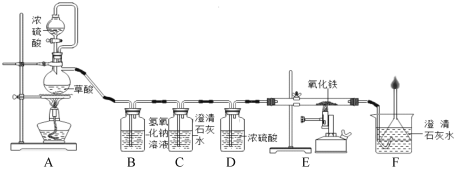
Aװ����ʵ�������ò��ᣨH2C2O4����Ũ���������ȡһ����̼�����巢��װ�ã���Ӧ�Ļ�ѧ����ʽ�ǣ�H2C2O4  H2O+CO2��+CO������ش��������⣺
H2O+CO2��+CO������ش��������⣺
��Cװ������������Cװ�õ�������_____________��
Dװ����Ũ�����������______________��
��Eװ���з�Ӧ�Ļ�ѧ����ʽ��________________��
��Fװ�õ�������___________________��
���𰸡���Ȼ�� C + H2O ![]() CO+H2 ͨ������£���Ȼ�������壬���Ӽ����ϴ����ױ�ѹ��������ȼ���ǹ��壬���Ӽ�����С�����ѱ�ѹ�� ABC �����������ж�����̼�Ƿ���� ����һ����̼ 3CO+Fe2O3
CO+H2 ͨ������£���Ȼ�������壬���Ӽ����ϴ����ױ�ѹ��������ȼ���ǹ��壬���Ӽ�����С�����ѱ�ѹ�� ABC �����������ж�����̼�Ƿ���� ����һ����̼ 3CO+Fe2O3![]() 2Fe+3CO2 ֤���ж�����̼���ɣ�ͬʱ���������һ����̼���壬��ֹ������Ⱦ
2Fe+3CO2 ֤���ж�����̼���ɣ�ͬʱ���������һ����̼���壬��ֹ������Ⱦ
��������
��1������ʹ�õĻ�ʯȼ����Ҫ����ú��ʯ�͡���Ȼ���������Ȼ��
��2��������ʾ��ͼ��̼��ˮ�ڸ���������һ����̼���������÷�Ӧ�Ļ�ѧ����ʽ��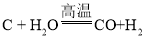 �����
�����
��3��Ϊ������������䣬��Ȼ��ͨ����ѹ�����ݻ���С�ĸ�ƿ�У�����ȼ��ȴ���ѱ�ѹ�����Դӷ��ӵĽǶȷ��������е�ԭ����ͨ������£���Ȼ�������壬���Ӽ����ϴ����ױ�ѹ��������ȼ���ǹ��壬���Ӽ�����С�����ѱ�ѹ�������ͨ������£���Ȼ�������壬���Ӽ����ϴ����ױ�ѹ��������ȼ���ǹ��壬���Ӽ�����С�����ѱ�ѹ����
��4����ȼ�����ŵ���Ҫ�Ǵ����ḻ��ȼ��ֵ����ࡢ����Ⱦ������ABC��
��5��
��Cװ���еij���ʯ��ˮ�������Ǽ����������ж�����̼�Ƿ������Dװ����Ũ����������Ǹ���һ����̼����������������ж�����̼�Ƿ����������һ����̼��
��Eװ���з�Ӧ�Ļ�ѧ����ʽ��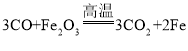 �����
�����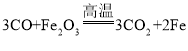
��Fװ�õ�������֤���ж�����̼���ɣ�ͬʱ���������һ����̼���壬��ֹ������Ⱦ��
���֤���ж�����̼���ɣ�ͬʱ���������һ����̼���壬��ֹ������Ⱦ��

����Ŀ��С�մ����Ҫ�ɷ���̼�����ƣ���ͼ1��һ��ijƷ��С�մ�ı�ǩ��Ϣ��
ѧУ��ѧ��ȤС���ͬѧȡ������С�մ���Ʒͨ��ʵ�����ⶨijϡ�������ʵ�����������ͬʱ�ⶨ��С�մ���Ʒ�ﵽ������������ȤС����Ĵ���ʢ��50gϡ������ձ������μ����������С�մ���ͼ1�������ձ�����Ϊ100g������ֽ�����ȡ���ӳ���ʾ�Ķ������������ݼ�¼���±���
���� | ��1�� | ��2�� | ��3�� | ��4�� |
����С�մ���Ʒ������/g | 5 | 5 | 5 | 5 |
���ӳ���ʾ�Ķ���/g | 152.4 | 154.8 | 157.2 | 162.2 |
���ϱ�������������¼��㲢�ش����⣨���ʲ������ᷴӦ��

��1������ͼ2�����л�������С�մ���Ʒ���������������������������ͼ_____��
��2������ϡ������������������Ƕ��٣�д��������̣���ȷ��0.1%��_____��
��3��С�մ���̼����������������99.5%Ϊ�ŵ�Ʒ���ﵽ98.2%Ϊһ��Ʒ��������жϸ�С�մ���Ʒ�ﵽ_____�������ŵ�Ʒ������һ��Ʒ���������ӱ�ǩ��Ϣ����֪������С�մ�ķ�����_____��