��Ŀ����
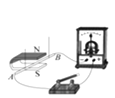
����Ŀ��ij�о���ѧϰС����������װ�ý����������ȡʵ�飬������ش��������⡣

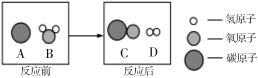
��1������������������_____��
��2��ѡ��Aװ���ø��������ȡ�����Ļ�ѧ����ʽ_____��A��E���ӣ���ȡ������������ԭ����_____��дһ�㼴�ɣ���
��3��ѡ��Bװ����п����ϡ������ȡ�����Ļ�ѧ����ʽ��_____��Ϊ�˿��Ƹ÷�Ӧ���ʣ��ɽ��������������_____��
��4������Dװ���ռ�������̼��������Ƿ��ռ����ķ�����_____������Fװ���ռ�������̼��������Ӧ��_____��ͨ�루�a����b����������Fװ�ü����Ƶõ������Ƿ�Ϊ������̼������Fװ���з���һ������_____��������Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡���1������©����
��2��2KMnO4![]() K2MnO4+MnO2+O2���������ܿڸ������ݾͿ�ʼ�ռ�������ƿ��ûװ��ˮ�Ⱥ����𰸣���
K2MnO4+MnO2+O2���������ܿڸ������ݾͿ�ʼ�ռ�������ƿ��ûװ��ˮ�Ⱥ����𰸣���
��3��Zn+H2SO4=ZnSO4+H2������Һ©����
��4����ȼ�ŵ�ľ�����ڼ���ƿ�ڣ���ľ��Ϩ��֤��������̼���ռ�����a������ʯ��ˮ��CO2+Ca��OH��2=CaCO3��+H2O.
��������
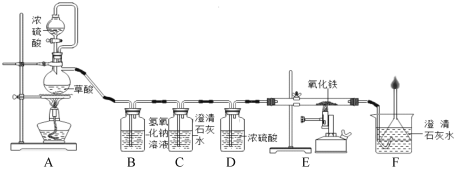
���⣨1��ͨ������©����������ƿ��ע��Һ��ҩƷ���������������dz���©����
��2������������ȷֽ���������ء��������̺�����������д����ѧ����ʽ���ɣ�����ˮ���ռ�����Ҫ��������������ð��ʱ��ʼ�ռ����Է��ռ����������������A��E���ӣ���ȡ�����������Ŀ���ԭ���ǣ����ܿڸ�������ð���Ϳ�ʼ�ռ������⼯��ƿ����ûװ��ˮ���������ݣ�Ҳ��ʹ�ռ������岻����
��3��ʵ���ҳ���п����ϡ���᳣���·�Ӧ��ȡ������������Ϊ����п���������ɷ�Ӧ����������ȡ��д��ѧ����ʽ��Ϊ�˿��Ƹ÷�Ӧ���ʣ�����©�����ɷ�Һ©����ע�����ȣ�
��4��ʵ������ȡ������̼�ô���ʯ��ϡ���ᷴӦ����Ϊ������̼һ�㲻ȼ��Ҳ��֧��ȼ�գ���ʹȼ�ŵ�ľ��Ϩ�𣬹�����������̼�ķ����ǽ�ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��۲�ľ���Ƿ�Ϩ������жϣ�����Fװ���ռ�������̼����Ϊ������̼�ܶȱȿ������������ſ������ռ���Ӧ�����̳�������Ӧ��a��ͨ�룻���������̼�ó���ʯ��ˮ������Fװ���з���һ�����ij���ʯ��ˮ��CO2+Ca��OH��2=CaCO3��+H2O.

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
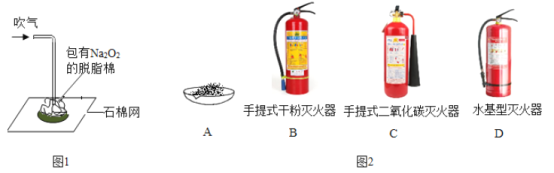
����ѵ��ϵ�д�����Ŀ���ڻ�ѧѧ�ƽ��ϣ���ѧ��ʦ��������������������ħ����������й������ƣ�Na2O2����ĩ����֬���������ܴ�������֬��ȼ�������ˣ���ͼ1����ͬѧ�Ƕ�ʵ�������Ũ�����Ȥ��
��1������ȼ����������֬���ǿ�ȼ�С����Ϊ�������ƣ�Na2O2���봵����CO2��H2O��Ӧ�����˿���֧��ȼ�յ����ʣ����һ�_____�����������������ų�����������������ȼ�յ���һ������_____��
��2����ȤС���ͬѧ����Ӧ��Ĺ������ˮ�У����ֹ���ȫ���ܽ��γ���ɫ��Һ�����ǣ�����Һ�����ʳɷֽ���̽����
���������ϣ�Na2O2�ǵ���ɫ���壬���������̼��Ӧ��Ҳ����ˮ��Ӧ���ֱ�����̼���ƺ��������ƣ����ų�������
��������룩����һ��������Na2CO3���������������NaOH����������������_____��
��ʵ����̽����
ʵ�鲽�� | ʵ������ | ʵ����� |
1��ȡ������Һ���Թ��У������еμӼ�����ɫ��̪���� | ��Һ��ɫ | ��������� |
2����ȡ��Ӧ����Һ�������Թ��У������еμ�ϡ���� | _____ | ����������� |
3��ȡ����1����Һ���Թ��У������еμ�������_____��Һ���� | ��_____��_____ | ����һ������ |
����չ�뽻����
��1��С֣ͬѧ��Ϊ����1��ʵ����۲���ȷ�������ԭ��_____��
��2������2��ʵ������˵����̼���ƵĴ��ڣ���Ӧ�Ļ�ѧ����ʽΪ_____��
��3��Na2O2Ҳ����ϡ���ᷴӦ�������Ȼ��ơ�ˮ����������Ӧ�Ļ�ѧ����ʽΪ_____��
��4�����������й������ƣ�һ��ʧ����ѡ��ͼ2�е����������_____������ĸ����
����Ŀ���Ķ�������ն��ġ�
ʯīϩ��Ŀǰ��֪������Ӳ�IJ��ϣ������۵�ߴ�3000�棬���������ĵ��硢�������ܡ�2004�꣬��ѧ�Ҵ�ʯī�а��������һ��̼ԭ�ӹ��ɵ�ʯī��Ƭ�������ʯīϩ��Ŀǰ���õ��Ʊ�����������ʯī��ԭ����ʯīƬ��Ũ���ᡢŨ���ᡢ������صȷ�Ӧ���پ����������õ�����ʯīϩ�����ͨ����ԭ�õ�ʯīϩ��
�ҹ��Ŀ����Ŷӷ�����ʯīϩ�ķ������ܣ���������صĶ�άƬ��ṹ����Ϳ���е����γ����ܸ����㣬ʹ��ʴ��������ͨ�����������������á���ͼ2�����������ʯīϩ��Ĥ��ͭ�ڸ�����Ҳû�б���ʴ����ʯīϩ��߷��Ӿۺ��︴���γɸ���Ϳ�ϣ�������Ϳ�ϵķ���ʴ���ܡ�
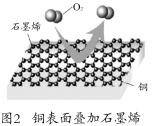
��1��ij����Ϳ�ϵĻ������ܣ�ͿĤ�����ͬ��
���� | ʯīϩ����Ϊ0 | ʯīϩ����Ϊ1% | ʯīϩ����Ϊ2% |
������ | 240h���쳣 | 240h���쳣 | 300h���쳣 |
�ͼ��� | 168h���쳣 | 168h���쳣 | 240h���쳣 |
���ϻ� | 60h��ɫ | 100h��ɫ | 1500h��ɫ |
��ʯīϩ��ص��¼������·�����õ�Խ��Խ�㷺��Ӧ�á�
�����������ݻش��������⡣
��1��ʯīϩ���е�����������______��дһ�㼴�ɣ���
��2��ʯīϩ����______������������������������������
��3��ʯīϩ���Է�����ԭ����______��
��4�����ݱ�1�������ø���Ϳ����ʯīϩ�ı���Ϊ______ʱ����������á�
��5�����й���ʯīϩ��˵����ȷ����______��
A ����ʯī��ԭ��ֻ�����������仯
B ʯīϩ����������ȫȼ�յIJ�����CO2
C �ڸ��������£�ʯīϩ�Ծ��з�������
D ����ʯīϩ�ĸ���Ϳ�Ϸ������ܸ���