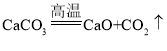��Ŀ����
����Ŀ����ѧ���ڷ��ӡ�ԭ�ӵIJ�����о����ʵ����ʡ���ɡ��ṹ��仯���ɵĿ�ѧ��
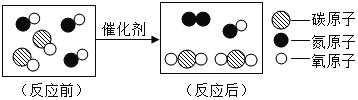
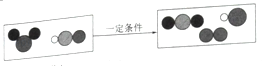
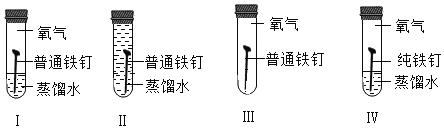
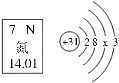
��1����ͼ������β���ڴ��������µľ�������ԭ������ʾ�Ļ�ѧ��Ӧ����ʽΪ_________�������й�˵������ȷ����_________
A ��ѧ��Ӧǰ��ԭ�ӵ��������Ŀ����
B ÿ��������̼������ 1��̼ԭ�Ӻ� 1�������ӹ���
C һ����̼���ӺͶ�����̼���Ӷ�����̼����ԭ�ӹ��ɵģ��ʻ�ѧ������ͬ
D �ɷ��ӹ��ɵ����ʷ�����ѧ��Ӧ��ʵ���Ƿ��ӷֳ�ԭ�ӣ�ԭ��������ϳ��·���
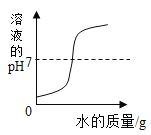
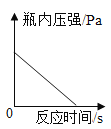
��2����ʢ�г���ʯ��ˮ���ձ��в���絼�ʴ��������������г���ͨ������Ķ�����̼���壬�۲쵽��Һ�ȱ���Ǻ��������ʧ����絼��������ͼ��
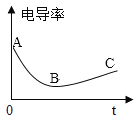
�����Ϣ���ٵ絼�ʴ��������ڲ�����Һ������ǿ������Һ�絼��Խ��λ�����Һ�������ƶ���������ĿԽ�࣬����Ũ��ҲԽ��CaCO3+H2O+CO2=Ca(HCO3)2��Ca(OH)2��CaCO3��Ca(HCO3)2�������ܽ�ȷֱ�Ϊ��0.165g��0.0005g��16.6g����ˮ�ĵ����Ժ������絼�ʽӽ�0��
������Ƕ�
��AB�η�����Ӧ�Ļ�ѧ����ʽ_________��
��B��絼�ʲ�Ϊ 0��ԭ����_________��
��BC�ε絼�����ߵ�ԭ����_________
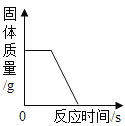
��3����ʢ��һ������ϡ����������Һ���ձ��еμ� 2~3 ����ɫ��̪��Һ��������һ֧�絼�ʴ������� Ȼ����εμ�ϡ���ᣬ�����Һ�ĵ絼�ʱ仯�����루2���м�Ϊ���ƣ�
��AB �η�����Ӧ�Ļ�ѧ����ʽ_____��
�ڹ۲쵽�а�ɫ������������Һ��ɫ�仯Ϊ_____��
��ʵ�ʲ��뷴Ӧ������_____��
��BC �ε絼�����ߵ�ԭ��____
���𰸡�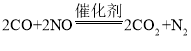 BC Ca(OH)2+CO2=CaCO3��+H2O ��Ȼ�������Ʊ�ת��Ϊ̼��ƣ���̼����Կ��� Ca2+��
BC Ca(OH)2+CO2=CaCO3��+H2O ��Ȼ�������Ʊ�ת��Ϊ̼��ƣ���̼����Կ��� Ca2+�� ![]() ����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�� ����ͨ�������̼����Ӧ�������ܵ�Ca(HCO3)2����Һ��Ca2+��
����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�� ����ͨ�������̼����Ӧ�������ܵ�Ca(HCO3)2����Һ��Ca2+��![]() ������Ũ�������� Ba(OH)2+H2SO4=BaSO4��+2H2O �ɺ�ɫ��Ϊ��ɫ H+��OH-��Ba2+��
������Ũ�������� Ba(OH)2+H2SO4=BaSO4��+2H2O �ɺ�ɫ��Ϊ��ɫ H+��OH-��Ba2+��![]() �����ϡ�����к��д�����H+��
�����ϡ�����к��д�����H+��![]() ��ʹ����Һ����Ũ������
��ʹ����Һ����Ũ������
��������
��1��������Ӧ���۹���ͼ��֪����Ӧǰ��1��̼ԭ�Ӻ�1����ԭ�ӹ��ɵ�CO��������1����ԭ�Ӻ�1����ԭ�ӹ��ɵ�NO���ӷ�����Ӧ����������2��Nԭ�ӹ��ɵ�N2���Ӻ���2��Oԭ����1��Cԭ�ӹ��ɵ�CO2���ӣ��÷�Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪ��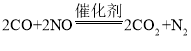 �����������غ㶨�ɿ�֪�κλ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䶼�������ı䣻ÿ��������̼������2��̼ԭ�Ӻ� 1����ԭ�ӹ��ɣ���������ԭ�ӹ��ɵģ����Ӳ����ɷ��ӹ��ɣ��ɷ��ӹ��ɵ����ʵĻ�ѧ�����ɷ��������֣�һ����̼�Ͷ�����̼�ķ��ӹ��ɲ�ͬ�����Ի�ѧ����Ҳ��ͬ���ɷ��ӹ��ɵ����ʷ�����ѧ��Ӧ��ʵ�ʾ����Ƿ��ӷֳ�ԭ�ӣ�ԭ��������ϳ��·��ӡ����
�����������غ㶨�ɿ�֪�κλ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䶼�������ı䣻ÿ��������̼������2��̼ԭ�Ӻ� 1����ԭ�ӹ��ɣ���������ԭ�ӹ��ɵģ����Ӳ����ɷ��ӹ��ɣ��ɷ��ӹ��ɵ����ʵĻ�ѧ�����ɷ��������֣�һ����̼�Ͷ�����̼�ķ��ӹ��ɲ�ͬ�����Ի�ѧ����Ҳ��ͬ���ɷ��ӹ��ɵ����ʷ�����ѧ��Ӧ��ʵ�ʾ����Ƿ��ӷֳ�ԭ�ӣ�ԭ��������ϳ��·��ӡ����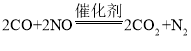 ��BC��
��BC��
��2����AB����Һ�ĵ�������С��˵����Һ���������ƶ��������ڱ��٣������������ƺͶ�����̼��Ӧ����̼��Ƴ�����ˮ���µģ���Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2+CO2=CaCO3��+H2O�����Ca(OH)2+CO2=CaCO3��+H2O��
��B��ʱ��ȻCa(OH)2ȫ����CO2ת����CaCO3����̼����Կ���Ca2+�� ![]() ����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�ȣ�����Һ���Ե��硣�����̼����Կ��� Ca2+��
����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�ȣ�����Һ���Ե��硣�����̼����Կ��� Ca2+�� ![]() ����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�ȡ�
����ʽ�����ܽ���ˮ�У�ά��һ��������Ũ�ȡ�
�����Ŷ�����̼�IJ���ͨ�룬������̼���̼����Լ�ˮ��Ӧ�������ܵ�Ca(HCO3)2����Һ��Ca2+��![]() ������Ũ��������ʹ��������ǿ���������ͨ�������̼����Ӧ�������ܵ�Ca(HCO3)2����Һ��Ca2+��
������Ũ��������ʹ��������ǿ���������ͨ�������̼����Ӧ�������ܵ�Ca(HCO3)2����Һ��Ca2+��![]() ������Ũ��������
������Ũ��������
��3����AB����Һ�ĵ�������С��˵����Һ���������ƶ��������ڱ��٣���������������ϡ���ᷴӦ�������ᱵ������ˮ���µģ���Ӧ�Ļ�ѧ����ʽΪ��Ba(OH)2+H2SO4=BaSO4��+2H2O�����Ba(OH)2+H2SO4=BaSO4��+2H2O��
���������������ᷴӦ�������ᱵ��ˮ����Һ�ɼ���������ԣ�������ĵμӵ�����ʱ�ֱ�����ԣ�����ֻ̪�����죬���Գ��˻ῴ�����ɰ�ɫ���������ῴ����Һ�ɺ�ɫ��Ϊ��ɫ������ɺ�ɫ��Ϊ��ɫ��
�����������е����������Ӻ�ϡ�����е������ӽ������ˮ�����������еı����Ӻ�ϡ�����е���������ӽ���������ᱵ�İ�ɫ����������ʵ�ʲμӷ�Ӧ�����ǣ�H+��OH-��Ba2+��![]() �����H+��OH-��Ba2+��
�����H+��OH-��Ba2+��![]() ��
��
��ϡ���������������ȫ��Ӧ������ϡ����ļ��룬ϡ�����к��д�����H+��![]() ��ʹ����Һ����Ũ���������Ծ���ǿ�ˡ���������ϡ�����к��д�����H+��
��ʹ����Һ����Ũ���������Ծ���ǿ�ˡ���������ϡ�����к��д�����H+��![]() ��ʹ����Һ����Ũ������
��ʹ����Һ����Ũ������

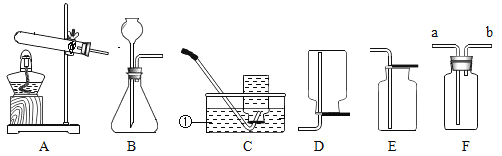
����Ŀ������ʵ���ܴ��ʵ��Ŀ�ĵ���
A | B | C | D | |
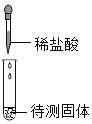
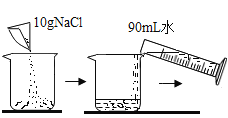
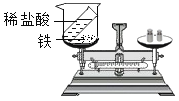
Ŀ�� | ����̼���� | ������������ 10%��NaCl ��Һ | ��֤�����غ㶨�� | ��֤�������Ӵ���ȼ�յ�����֮һ |
ϡ���� | ||||
ʵ�� |
|
|
|
|
A.AB.BC.CD.D