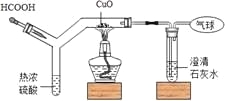
��Ŀ����
����Ŀ���������Ʊ��治�����ױ��ʡ�Ϊ�ⶨһƿ�����������ƵĴ��ȣ�ȡ����������ͼ��ʾ��ʵ�飬�����ͼʾ�����ݷ������㣺���ٶ�����ȫ���ݳ���������ˮ������������Ļӷ���


��1����Ӧ���������������̼������Ϊ g��
��2�������Ʒ���������Ƶ�����������д��������̣������ȷ��0.1%����
��3��ʵ��ǰ���ձ��и�Ԫ�ص����� �����������С�����䡱����
���𰸡���1��2.2����2��59.7%����3������
������������ͼ��Ӧ�Ļ�ѧ����ʽ���
�⣺��1����ͼ��֪����Ӧ���������������̼������Ϊ��2.2g��
��2������Ʒ��̼��Ƶ�����Ϊx��
CaCO3 + 2HCl == CaCl2 + H2O + CO2��
100 44
x 2.2g
![]()
x=5g
��Ʒ���������Ƶ�����Ϊ12.4g-5g=7.4g��
��Ʒ���������Ƶ���������=![]() =59.7%��
=59.7%��
��3�����������غ㶨�ɿ�֪����Ӧǰ��Ԫ���������䡣��ʵ��ǰ���ձ��и�Ԫ�ص��������䡣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ