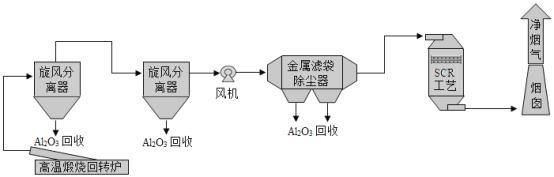
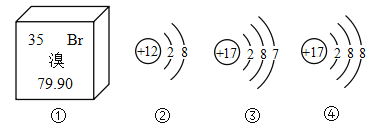��Ŀ����
����Ŀ��ÿ���������ʴ��ɵľ�����ʧԶ��������Ȼ�ֺ���ɵ���ʧ�ܺ͡������������� ��������һ�ֳ����ķ����ֶΡ�
��.�����㲿�����淢��
��ҵ�ϳ�������������з���������ʹ������γ�һ�����ܵ�����ɫ����Ĥ���������£�
��1���������ɰֽ��ĥ�����̷�������_____�����������仯��������ѧ�仯������
��2����������ڼ��������½��У����ȵ�������___���������ʱ���ó���Һ ��ϡ���ᣩ����ʱ�䲻��̫����ԭ����_____���û�ѧ����ʽ��ʾ����
��3��Ϊ���鲽���������������Ƿ�ϸ�����Ʒ������� 5%������ͭ��Һ��������δ�γ����ܵ�����Ĥ��һ��ʱ��۲쵽������Ϊ______��
��.ͭ�������߱������
��4��ѡ��ͭ�����ߵ���Ҫԭ����______��Ȼ��ͭ���⽫���ӵ��裬����ʹ�����������ڿ����б�����γ����ܵĶ���������SnO2�������������������ᷢ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ_______��
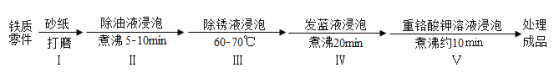
��5�������������IJ�����Ϣ��
�۸� | �۵� | �������۵� | |
�� | 133000 Ԫ/�� | 232�� | 1127�� |
�� | 12770 Ԫ/�� | 660�� | 2054�� |
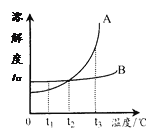
�ɱ�����Ϣ��֪�����ļ۸�Զ��������ѡ����ͭ�����������������Ҫ��Ϊ�����۵���ͣ����������ߵ�������˵���۵�͵���������_______��
��6����ȡij�Ͼ������ߣ�ȷ��ȡһ������Ʒ�������������н��ݣ��۲쵽____����Һ��Ϊ��ɫ����ȫ��Ӧ��ʣ�����ȡ����ϴ����____������������ʵ������¼���£������н���ͭ����������Ϊ_________���������������ٷֺ���С�����һ λ����
ʵ �� | 1 | 2 | 3 |
��Ʒ����/g | 0.5529 | 0.5659 | 0.5860 |
ʣ���������/g | 0.5291 | 0.5419 | 0.5604 |
���𰸡������仯 �ӿ췴Ӧ ![]() ��������к�ɫ�������� �����Ժ�
��������к�ɫ�������� �����Ժ� ![]() ������ ����һ��ʱ������������ݲ�����ֻ�ش��������ݲ����������֣� ��ɻ���� 95.7%
������ ����һ��ʱ������������ݲ�����ֻ�ش��������ݲ����������֣� ��ɻ���� 95.7%
��������
��1����ɰֽ��ĥ��������û�����������ʣ����������仯��
��2����������ڼ��������½��У����ȵ������Ǽӿ췴Ӧ���������������ᷴӦ�����ԣ��������ᴦ�������������ʱ������ʱ�䲻�˹����������������ܽ⣬�������ᷴӦ����������������������Ӧ�Ļ�ѧ����ʽΪ��Fe+H2SO4�TFeSO4+H2����
��3�����������Ʒ���ϸ�������������ͭ��Ӧ��������������ͭ���ʱ������ֺ�ɫ����������
��4��ѡ��ͭ����������Ϊͭ�����Ժã����������������ᷢ�����ֽⷴӦ����Ӧ�������Ȼ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
��5�����������ߵ�������˵���۵�������ӣ�
��6���Ͼ������߱���������Ĥ���������������н��ݣ���۲쵽����һ��ʱ������������ݲ�������ȫ��Ӧ��ʣ�����ȡ����ϴ������ɡ��ٳ����������н���ͭ����������Ϊ![]() ��
��

����Ŀ��ij��ѧ��ȤС���ͬѧ������������̼��ʵ������ȡ��������ʵ��ʱ�����ֳ�ʱ�������ʯ��ˮ��ͨ�������̼��ʯ��ˮ�ȱ���ǣ����ֱ���壬���ȳ����Һ���ֱ���ǡ������ʦ��֪�������ܵ�̼������������̼��ˮ��Ӧ���������ܽ��̼����ƣ�CaCO3+CO2+H2O=Ca(HCO3)2��̼����������ֽ⣺Ca(HCO3)2 ![]() CaCO3��+H2O+CO2�������ǶԳ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
CaCO3��+H2O+CO2�������ǶԳ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
��������⣩
һ����CO2��NaOH��Һ��Ӧ�����Һ�����������ʲô?
���������ϣ�
��1��ͨ������CO2����Ӧ�Ļ�ѧ����ʽΪ�� __________________��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O=2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��������룩
��1������ΪNa2CO3��
��2������ΪNaHCO3��
��3������ΪNaOH��Na2CO3��
��4������Ϊ___________���ѧʽ����
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
A. ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | ��____���� | ���루2�������� |
B. ȡ����a�е��ϲ���Һ������ϡ���� | ������ð�� | ���루1���ͣ�3�������� |
���ó����ۣ�
���루4��������
�����۽�����
��д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ�� ___________��