��Ŀ����
ʯ��ʯ��һ�ֳ����Ŀ�ʯ������Ҫ�ɷ���̼��ƣ�CaCO3������ʯ��ʯΪԭ���ƵõIJ�Ʒ��Ӧ��ʮ�ֹ㷺�����̼��������ǵ�����������ϵ��
��1���Ϸ��ݰ�ɫ����ǽ��ˢ����Ҫ�ɷ���̼��ƣ�����йز��ŷ���һЩ�Ͻ�������ǽ�ķ�ˢ������ϰ�ʴ������Ϊ������һ�����ԭ������� ��
��2���ճ������г��õ������ﶼ����һ������Ħ��������������Ʋ⣬Ħ����Ӧ�߱������� ������ĸ����
A��������ˮ B��������ˮ C����Ӳ D������ E�������ϴ� F��������С
����ΪĦ������Ӧ�߱������ʻ��ص��� ��ֻ���һ�㣩��
����Ħ�����е�̼��ƿ���ʯ��ʯ����ȡ���Ż�ͬѧ�������ͼ1��ʾ��ʵ�鷽�����Ʊ�̼��ƣ�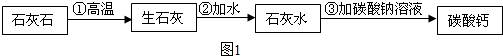
С���ϣ���Na2CO3+Ca��OH��2=2NaOH+CaCO3����̼���ƹ�ҵ��Ʒ�ۼ�2000Ԫ/�֣�
����ʯ�ҵ���Ҫ�ɷ��������ƣ�CaO��
��д�����������в���ڵĻ�ѧ��Ӧ����ʽ�� ��
�ͬѧ����ʯ��ʯΪԭ�ϣ��������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼ2��
���Ϊ�Լ���Ƶķ������Ż����ã����ŵ��� �������������Ƿ���̼��Ƶ�ʵ�鷽���� ��
��3��������һ���������ҩ�ģ�������̼��ơ������ʡ�ˮ�ݡ�ʮ���ְ�����Ͷ�����Ԫ�أ�ij��ȤС��������ѧ���Ļ�ѧ֪ʶ������ͼ��3ʾ��ʵ����̣����ⶨ����ijƷ���������̼��Ƶ�������������̼����⣬�������ʲ������ᷴӦ����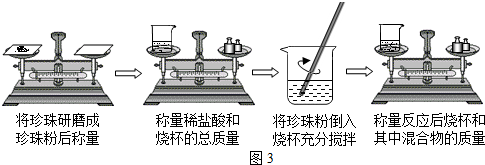
��ijͬѧ��Ϊ��������ʵ������У��ò��������Ͻ��裬��ʹ�ⶨ����С���������� ��
��������ʵ���н�������ĥ�ɷ۵�Ŀ���� ��
�۲������ݼ�¼���£�ֻ����̼��������ᷴӦ��������������
��������������жϣ� �飨��ס����ҡ������������Բ���������ʵ���ã�
���������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼����Ϊ g��
���������������̼��Ƶ�����������д��������̣���
��1���Ϸ��ݰ�ɫ����ǽ��ˢ����Ҫ�ɷ���̼��ƣ�����йز��ŷ���һЩ�Ͻ�������ǽ�ķ�ˢ������ϰ�ʴ������Ϊ������һ�����ԭ�������
��2���ճ������г��õ������ﶼ����һ������Ħ��������������Ʋ⣬Ħ����Ӧ�߱�������
A��������ˮ B��������ˮ C����Ӳ D������ E�������ϴ� F��������С
����ΪĦ������Ӧ�߱������ʻ��ص���
����Ħ�����е�̼��ƿ���ʯ��ʯ����ȡ���Ż�ͬѧ�������ͼ1��ʾ��ʵ�鷽�����Ʊ�̼��ƣ�

С���ϣ���Na2CO3+Ca��OH��2=2NaOH+CaCO3����̼���ƹ�ҵ��Ʒ�ۼ�2000Ԫ/�֣�
����ʯ�ҵ���Ҫ�ɷ��������ƣ�CaO��
��д�����������в���ڵĻ�ѧ��Ӧ����ʽ��
�ͬѧ����ʯ��ʯΪԭ�ϣ��������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼ2��

���Ϊ�Լ���Ƶķ������Ż����ã����ŵ���
��3��������һ���������ҩ�ģ�������̼��ơ������ʡ�ˮ�ݡ�ʮ���ְ�����Ͷ�����Ԫ�أ�ij��ȤС��������ѧ���Ļ�ѧ֪ʶ������ͼ��3ʾ��ʵ����̣����ⶨ����ijƷ���������̼��Ƶ�������������̼����⣬�������ʲ������ᷴӦ����

��ijͬѧ��Ϊ��������ʵ������У��ò��������Ͻ��裬��ʹ�ⶨ����С����������
��������ʵ���н�������ĥ�ɷ۵�Ŀ����
�۲������ݼ�¼���£�ֻ����̼��������ᷴӦ��������������
| ��� | ��Ӧǰ | ��Ӧ�� | |
| �ձ���ϡ��������� | ����۵����� | �ձ������л��������� | |
| �� | 150.0g | 2.8g | 151.7g |
| �� | 150.0g | 0.28g | 150.17g |
���������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼����Ϊ
���������������̼��Ƶ�����������д��������̣���
���㣺�εĻ�ѧ����,ʵ��̽�����ʵ���ɳɷ��Լ�����,֤��̼����,̼��ơ���ʯ�ҡ���ʯ��֮���ת��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�������� ��ѧ����,��ѧ̽��
��������1�����ݷ������ڵصĿ����к��н϶�Ķ��������γɵ�������ɵĽ��з�����
��2������������Ħ���������ý��з�����
���������������Ӵ����з�����
�������ַ����еķ�Ӧ���������з�����
����ʵ���Ҽ���̼���εķ������з�����
��3���ٸ��ݽ�����Լ��ٷ�Ӧ�ķ������з�����
�ڸ�������Ӧ��ĽӴ���������Լӿ췴Ӧ�����ʽ��з�����
�۸������е����ݺͻ�ѧ����ʽ���з�����
��2������������Ħ���������ý��з�����
���������������Ӵ����з�����
�������ַ����еķ�Ӧ���������з�����
����ʵ���Ҽ���̼���εķ������з�����
��3���ٸ��ݽ�����Լ��ٷ�Ӧ�ķ������з�����
�ڸ�������Ӧ��ĽӴ���������Լӿ췴Ӧ�����ʽ��з�����
�۸������е����ݺͻ�ѧ����ʽ���з�����
����⣺��1��̼��ơ�ˮ�Ͷ�����̼������̼����ƣ�̼����ƻ�����ˮ�У�����ԭ������ǣ��������ڵصĿ����к��н϶�Ķ��������γɵ�������ɵģ�
��2�������е�Ħ����������Ħ����ȥ���ݱ�����۹��ģ�����Ħ����Ӧ�߱������У�B��C��F��
Ħ�������˵Ŀ�ǻ�Ӵ������������ģ�����Ħ������Ӧ�߱������ʻ��ص��У�����
����ڵķ�Ӧ�������ƺ�ˮ��Ӧ�����������ƣ���ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
�����Ʒ����У�������̼�õ��˳�ֵ����ã����Կ��Խ�Լ�ɱ���
��ʵ���Ҽ���̼���γ������ᣬȻ�����ɵ�����ͨ�����ʯ��ˮ�м������ɵ����壬���Լ����������Ƿ���̼��Ƶ�ʵ�鷽���ǣ�ȡ��Ʒ���������ᣬ�����ݲ����������ɵ�����ͨ�����ʯ��ˮ���ְ�ɫ������֤�������к���̼��ƣ�
��3���ٽ�������Ƿ�Ӧ���ٷ�����Ҳ��С�˶�����̼���ܽ����������������ǣ����Ͻ��裬��ʹ������������ַ�Ӧ��ͬʱ�����ڶ�����̼��ɢ�ݣ�
����ĥ�ɷ������˷�Ӧ��ĽӴ�������ӿ��˷�Ӧ�����ʣ����Խ�������ĥ�ɷ۵�Ŀ���ǣ�����������ĽӴ������ʹ�����е�̼�����ȫ��Ӧ��
��������ƽ���������е���С�ֶ�ֵ��֪����ƽ�����Ľ����С�������һλ������������������Բ���������ʵ���ã�
�������ɵĶ�����̼����������150+2.8-151.7=1.1g��
�������ɵĶ�����̼����������150+0.28-150.17=0.11g��
���Ը��������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼����Ϊ��1.1��0.11g��
�⣺��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x ��152.8g-151.7g��=1.1g
100��44=x��1.1g
��ã�x=2.5g
��100%=89.3%
���������̼��Ƶ���������ԼΪ89.3%��
�ʴ�Ϊ����1���������ڵصĿ����к��н϶�Ķ��������γɵ�������ɵģ�
��2��B��C��F������CaO+H2O=Ca��OH��2����Լ�ɱ���ȡ��Ʒ���������ᣬ�����ݲ����������ɵ�����ͨ�����ʯ��ˮ���ְ�ɫ������֤�������к���̼��ƣ�
��3���ٲ��Ͻ��裬��ʹ������������ַ�Ӧ��ͬʱ�����ڶ�����̼��ɢ�ݣ�
������������ĽӴ������ʹ�����е�̼�����ȫ��Ӧ��
���ң�1.1��0.11
�⣺��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x ��152.8g-151.7g��=1.1g
100��44=x��1.1g
��ã�x=2.5g
��100%=89.3%
���������̼��Ƶ���������ԼΪ89.3%��
��2�������е�Ħ����������Ħ����ȥ���ݱ�����۹��ģ�����Ħ����Ӧ�߱������У�B��C��F��
Ħ�������˵Ŀ�ǻ�Ӵ������������ģ�����Ħ������Ӧ�߱������ʻ��ص��У�����
����ڵķ�Ӧ�������ƺ�ˮ��Ӧ�����������ƣ���ѧ����ʽΪ��CaO+H2O=Ca��OH��2��
�����Ʒ����У�������̼�õ��˳�ֵ����ã����Կ��Խ�Լ�ɱ���
��ʵ���Ҽ���̼���γ������ᣬȻ�����ɵ�����ͨ�����ʯ��ˮ�м������ɵ����壬���Լ����������Ƿ���̼��Ƶ�ʵ�鷽���ǣ�ȡ��Ʒ���������ᣬ�����ݲ����������ɵ�����ͨ�����ʯ��ˮ���ְ�ɫ������֤�������к���̼��ƣ�
��3���ٽ�������Ƿ�Ӧ���ٷ�����Ҳ��С�˶�����̼���ܽ����������������ǣ����Ͻ��裬��ʹ������������ַ�Ӧ��ͬʱ�����ڶ�����̼��ɢ�ݣ�
����ĥ�ɷ������˷�Ӧ��ĽӴ�������ӿ��˷�Ӧ�����ʣ����Խ�������ĥ�ɷ۵�Ŀ���ǣ�����������ĽӴ������ʹ�����е�̼�����ȫ��Ӧ��
��������ƽ���������е���С�ֶ�ֵ��֪����ƽ�����Ľ����С�������һλ������������������Բ���������ʵ���ã�
�������ɵĶ�����̼����������150+2.8-151.7=1.1g��
�������ɵĶ�����̼����������150+0.28-150.17=0.11g��
���Ը��������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼����Ϊ��1.1��0.11g��
�⣺��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x ��152.8g-151.7g��=1.1g
100��44=x��1.1g
��ã�x=2.5g
| 2.5g |
| 2.8g |
���������̼��Ƶ���������ԼΪ89.3%��
�ʴ�Ϊ����1���������ڵصĿ����к��н϶�Ķ��������γɵ�������ɵģ�
��2��B��C��F������CaO+H2O=Ca��OH��2����Լ�ɱ���ȡ��Ʒ���������ᣬ�����ݲ����������ɵ�����ͨ�����ʯ��ˮ���ְ�ɫ������֤�������к���̼��ƣ�
��3���ٲ��Ͻ��裬��ʹ������������ַ�Ӧ��ͬʱ�����ڶ�����̼��ɢ�ݣ�
������������ĽӴ������ʹ�����е�̼�����ȫ��Ӧ��
���ң�1.1��0.11
�⣺��CaCO3������Ϊx
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x ��152.8g-151.7g��=1.1g
100��44=x��1.1g
��ã�x=2.5g
| 2.5g |
| 2.8g |
���������̼��Ƶ���������ԼΪ89.3%��
�������ڽ������ʱ�����ȷ������еķ�Ӧԭ����Ȼ��������Ϣ�Ϳα�֪ʶ����ϵ������ÿ��������Ŀ�Ļ�Ӧʵ�ʼ��ɽϿ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
С���ڼ�����ˮ�ƾ������������Ϊ70%�ľƾ���Һ10mL������ȡ7mL��ˮ�ƾ�ʱ��������Ͳ��Һ����ʹ�������7mL����ȡ3mL����ˮʱ��������Ͳ��Һ����ʹ�������3ml���������Ƶľƾ��У�������
| A����ˮ�ƾ�ȡ���� |
| B������ˮȡ���� |
| C�����һ������10mL |
| D�����һ��С��10mL |
�ڻ�ѧʵ�������в�����ȷ���ǣ�������
| A��Ϊ�˽�ԼҩƷʵ����ʣ���ҩƷ����ԭ�Լ�ƿ�� |
| B���ռ�CO2ʱ���Բ��������ſ����� |
| C����ʵ�����������û��Σ����ҩƷ����Ʒ�� |
| D�����������ƾ���ʹʵ����������ȼ��ʱ������ˮ���� |
��A��B��C��D�������ʷ����ܱ������м��ȣ���ѧ��Ӧǰ���������ʵ�������ϵ������ʾ��
�����ж���ȷ���ǣ�������
| A | B | C | D | |
| ��Ӧǰ/g | 4 | 16 | 111 | 4 |
| ��Ӧ��/g | X | 20 | 0 | 89 |
| A���÷�ӦΪ���Ϸ�Ӧ |
| B��X��ֵΪ26 |
| C��X��ֵΪ22 |
| D����Ӧ��A��B��C��D�������ʵ�����֮��Ϊ26��4��111��85 |
 ����Ԫ�ط���ΪBr����ͼ��x=
����Ԫ�ط���ΪBr����ͼ��x=