��Ŀ����
����Ŀ��ͬѧ����ʵ������һ���۲���ƾ����ڵľƾ������ý�ͷ�ιܴӾƾ�����ȡ�������ƾ����������У��û���ȼ�ƾ�����������ɫ���棬Ȼ���ڻ����Ϸ���һ�����������ձ���һ�ᣬ�����ձ��ڱ���Сˮ����֣���ת�ձ�������ע����������ʯ��ˮ������ʯ��ˮ����ǡ�
��1�����������۲쵽�ľƾ�����������_____��
��2��д���ƾ�����ѧʽC2H6O��ȼ�ջ�ѧ����ʽ_____��
���𰸡���ɫҺ�壬�����������ζ C2H6O+3O2![]() 2CO2+3H2O
2CO2+3H2O
��������
��1������Ҫ������ѧ�仯���ܱ��ֳ����������������ʵ��������ʣ��۲쵽�ľƾ���������������ɫҺ�壬�����������ζ�������ɫҺ�壬�����������ζ
��2����ʵ�������֪���ƾ��������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ�����C2H6O+3O2![]() 2CO2+3H2O
2CO2+3H2O

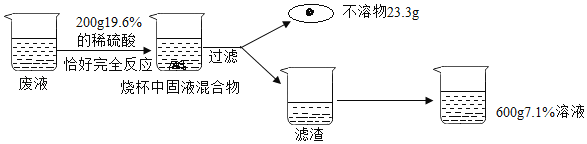
����Ŀ��Ϊ�ⶨij����ͭ��ͭ�Ĺ�������������ͭ������������С��ͬѧȡ20g�������������ձ��У���100gϡ�����Ϊ�ĵȷ����μ������н���ʵ�飬����������£�
���� | �� | �� | �� | �� |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 16 | a | 10 | 10 |
�ش��������⣺
��1��ԭ���������У�����ͭ����������Ϊ_____��
��2���ϱ��У�a��ֵΪ_____��
��3�������ʵ������ϡ���������ʵ�����������_____��д��������̣������ȷ��0.1%��