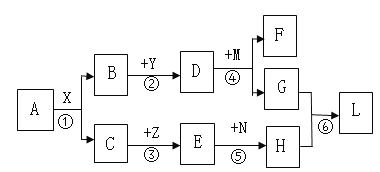
��Ŀ����
����Ŀ�������������������о��й㷺��Ӧ�á�
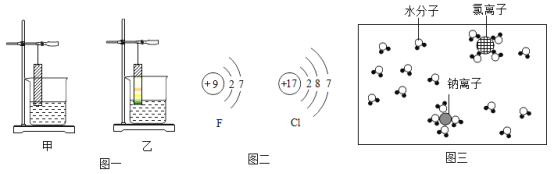
��1��д����ϡ�����ȥ����Ʒ��������Ļ�ѧ����ʽ_____��
��2��Ӧ�ý������˳���ܰ������ǽ�һ��ѧϰ�������ʡ���֪���и������ʼ���ܷ�����ѧ�� Ӧ��
A Fe �� CuSO4 ��Һ B Mg �� MnSO4 ��Һ C Mn �� FeSO4 ��Һ
��д�� Fe �� CuSO4 ��Һ��Ӧ�Ļ�ѧ����ʽ _____��
���������ֽ����Ļ����ǿ������˳����_____��
��3����ѧС��Ժ�������ͭ������п�ķ�Һ���������´�����
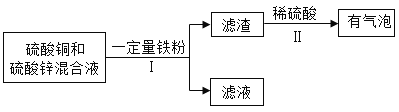
�ٲ������еIJ���������_____��
����Һ��һ�����е���������_____�����ѧ���ţ�
���𰸡�Fe2O3+3H2SO4�TFe2��SO4��3+3H2O Fe+CuSO4�TFeSO4+Cu Mg��Mn��Fe��Cu ���� Zn2+��Fe2+
��������
��1������Ʒ������������Ҫ�ɷ���������������ϡ���ᷴӦ������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3H2SO4�TFe2��SO4��3+3H2O�����Fe2O3+3H2SO4�TFe2��SO4��3+3H2O��
��2����Fe���Խ�Cu��CuSO4��Һ���û���������������ͭ��Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu�����Fe+CuSO4�TFeSO4+Cu��
�ڸ���������Ϣ����֪�����������Ļ��˳��Ϊ��Mg��Mn��Fe��Cu�����Mg��Mn��Fe��Cu��
��3���ٸ���ʵ��������з�����֪���������ǹ��ˡ�������ˡ�
���ڽ������˳���У�Zn��Fe��Cu����������ͭ������п����Һ�м������ۣ�ֻ����������ͭ��Һ��Ӧ��������������ͭ����������ϡ���������������˵��������������������һ������ͭ��������Һ��һ����������п��������������û������ͭ�������Һ��һ�����е���������Zn2+��Fe2+�����Zn2+��Fe2+��