��Ŀ����
ijͬѧ��������ͼ��ʾ��ʵ�飬��14��6%��ϡ�����м���̼��ƣ������10��6%��̼������Һ
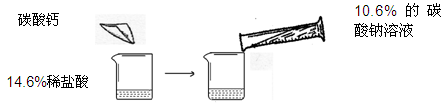
���ڶ�����������ǡ����ȫ��Ӧ����Һ��ʧ���Բ��ƣ�����ش���������
д��ʵ��һ�з�����ѧ��Ӧ�ķ���ʽ
��һ��ʵ���м���̼��ƺ���Һ�����ʳɷ�
������֪�����г����ڶ���ʵ�����ɳ����������ı���ʽ
ʵ���м���ϡ����m������Ϊ
������������������29��2%��Ũ��������ʵ��������ϡ���ᣬ����Ҫ��ˮ������
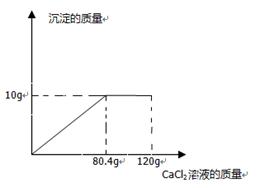
���ڶ��η�Ӧ�����Һ����191��2gˮ�������ò�������Һ�����ʵ���������Ϊ ��

| | ��һ�� | �ڶ��� |
| 14��6%��ϡ��������� | m | m |
| ����̼��Ƶ����� | 10g | 20g |
| ����10��6%��̼������Һ������ | 100g | 200g |
| ����̼������Һ��ʵ������ | ֻ������ | ֻ�а�ɫ���� |
д��ʵ��һ�з�����ѧ��Ӧ�ķ���ʽ
��һ��ʵ���м���̼��ƺ���Һ�����ʳɷ�
������֪�����г����ڶ���ʵ�����ɳ����������ı���ʽ
ʵ���м���ϡ����m������Ϊ
������������������29��2%��Ũ��������ʵ��������ϡ���ᣬ����Ҫ��ˮ������
���ڶ��η�Ӧ�����Һ����191��2gˮ�������ò�������Һ�����ʵ���������Ϊ ��
��1��CaCO3+2HCl=CaCl2+H2O+CO2����2���Ȼ��ơ��Ȼ��⣨3��106��100=21��2g:X ��4��100g ��5�� 100g ��6�� 23��4%
�����������1��ʵ��һ��ϡ�����̼��Ʒ�Ӧ������ʽΪCaCO3+2HCl=CaCl2+H2O+CO2����2����һ��ʵ�����̼������Һ��������ݣ�����ϡ�������������2����һ��ʵ���м���̼��ƺ���Һ�����ʳɷ�Ϊ���Ȼ��ƺ������3�������ڻ�ѧ��Ӧ�и����������ɱ��������Եڶ���ʵ�����ɳ����������ı���ʽΪ��106��100=21��2g:X��4��ǡ����20g̼��Ʒ�Ӧ��Ҫ���������Ϊ14��6g��������Ҫ������Һ������Ϊ��14��6g/14��6%=100g;��5����������������29��2%��Ũ��������ʵ��������ϡ���ᣬ������Һϡ��ǰ�����ʵ��������䣺����29��2%��Ũ��������ʵ�������14��6%��ϡ���ᣬ����Ҫ��ˮ������ΪX����200g*14��6%=��200g-X��*29��2% ��ã�X=100g����6�����ڶ��η�Ӧ�����Һ����191��2gˮ�������ò�������Һ�����ʵ���������Ϊ��23��4g/(200g+100g-8��8g-191��2g)*100%=23��4%��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ