��Ŀ����
����Ŀ��ˮ����Һ��������������������Ҫ�����á�
(1) ����������ˮ�в����γ���Һ����___________(�����)��
A������ B������ C��ʳ�� D���������
(2) �ס��ҡ������ֹ���������ˮ�е��ܽ��������ͼ��ʾ��
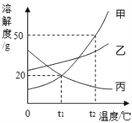
�����¶����ߣ��ܽ�ȼ�С��������_______________��
��t1��ʱ���ֱ���100gˮ�м���20g��20g�ң����γɱ�����Һ����__________��
�������й�������ȷ����__________(�����)��
A��t2��ʱ���ı�����Һ�����ʵ���������Ϊ50%
B���ֱ��ҵı�����Һ��t2�潵��t1��ʱ���������������Ϊ��>��
C���ӽӽ����͵ļ���Һ������һ�����ļ����ʣ����Һ����������������С
D��t2��ʱ���ֱ�50g�ס��ҡ�����������ˮ����ɱ�����Һ����Ҫˮ������Ϊ��<��<��
���𰸡� B �� �� D
��������(1) ��Һ�Ǿ�һ���ȶ��Ļ�������Һ�и������Է��ӡ�ԭ�ӵ������ӵ���ʽ���ϡ�A�������Է�����ʽ��ɢ��ˮ�У��γɵ�����Һ�� B���������͵η�ɢ��ˮ�У��γɵ�������Һ�� C��ʳ���������ӡ������ӷ�ɢ��ˮ�У��γɵ�����Һ�� D����������Լ����Ӻ�����ظ����ӷ�ɢ��ˮ�У��γɵ�����Һ����ѡB��(2) �����ܽ������ͼ�ϣ����������¶ȣ����������ܽ�ȡ����¶����ߣ��ܽ�ȼ�С�������DZ������ܽ����һ���¶��£�100g�ܼ���ﵽ����ʱ�����ܽ�����ʵ�������t1��ʱ�����ܽ��Ϊ20g,�ҵ��ܽ�ȴ���20g ���ֱ���100gˮ�м���20g��20g�ң����γɱ�����Һ���Ǽף���A�� ![]() ��t2��ʱ�����ܽ��Ϊ50g��������Һ�����ʵ���������ΪС��50%������B���ֱ������ļ��ҵı�����Һ��t2�潵��t1��ʱ���������������Ϊ��>�ң�����C�����ú��������ܼ��ķ���ʹ�ӽ����͵ļ���Һ������һ�����ļ����ʣ����Һ�Ǹ��¶��µı�����Һ�����������������������ý���ʹ���������ķ��������ʵ������������С������D��t2��ʱ���ס��ҡ������ܽ�ȴ�С��ϵ�Ǽ�>��>�����ֱ�50g�ס��ҡ�����������ˮ����ɱ�����Һ����Ҫˮ������Ϊ��<��<������ȷ����ѡD��
��t2��ʱ�����ܽ��Ϊ50g��������Һ�����ʵ���������ΪС��50%������B���ֱ������ļ��ҵı�����Һ��t2�潵��t1��ʱ���������������Ϊ��>�ң�����C�����ú��������ܼ��ķ���ʹ�ӽ����͵ļ���Һ������һ�����ļ����ʣ����Һ�Ǹ��¶��µı�����Һ�����������������������ý���ʹ���������ķ��������ʵ������������С������D��t2��ʱ���ס��ҡ������ܽ�ȴ�С��ϵ�Ǽ�>��>�����ֱ�50g�ס��ҡ�����������ˮ����ɱ�����Һ����Ҫˮ������Ϊ��<��<������ȷ����ѡD��

����Ŀ��������ͼ�ش�A��B�������е�һ�飬����ȫ�����𣬰�����һ��Ƿ֡�
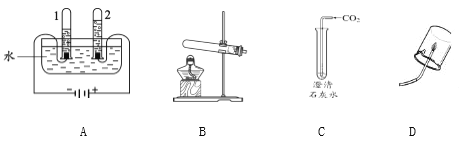
A | B |
��1��Aʵ����Թ�1�в�����������_______�� ��2��B��������������Ӧ�Ļ�ѧ����ʽΪ_____�� | ��1��C�й۲쵽��ʵ��������______�� ��2��Dʵ��ó��Ľ�����_______�� |