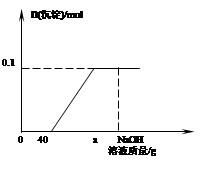题目内容
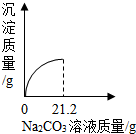
(5分)现有两种稀溶液:标记为A的0.0400%的氢氧化钠溶液;标记为B的0.365%的盐酸。假设本题所涉及到的各种稀溶液的密度均近似为1.00g·mL-1,且每滴溶液的体积近似为0. 05mL,试解答下列各小题。
(1)恰好完全中和20. 0gA溶液,需加入B溶液多少克?
(2)在盛有20. 0mLA溶液的锥形瓶中滴加2滴酚酞试液,再向瓶中缓缓倒人10. 0mLB溶液,边倒边振荡,充分混合后溶液呈无色。若取该无色混合液3.00mL于一支试管内,再向试管内滴加1滴A溶液,试通过计算说明此时试管内溶液呈现的颜色。
(1)2.00g; (2)溶液显无色
解析试题分析:已知量:氢氧化钠:20.0g×0.0400%=0.008g; 0.365%的盐酸;10. 0mLB溶液中氯化氢的质量;10. 0mLB溶液;混合液3.00mL;未知量:(l)恰好完全中和20. 0gA溶液,需加入B溶液的质量;(2)试管内溶液的颜色;
解:(1)恰好完全中和20. 0gA溶液,需加入B溶液的质量为x
HCl + NaOH==NaCl+H2O
36.5 40
0.365%x 0.008g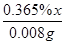 =
=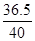
x=2.00g
(2)20. 0mL氢氧化钠溶液中含有氢氧化钠质量为8g;因为恰好完全中和20. 0gA溶液,需0.365%的盐酸的质量为2.00g,3mL盐酸中氯化氢的质量为:3mL·30mL-1×8g×0.365%=2.92g×10-3g;
设1滴A溶液中的氢氧化钠能中和1氯化氢的质量为y,
HCl + NaOH==NaCl+H2O
36.5 40
y 0.05g×0.04%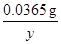 =
=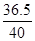
y-1.825g×10-3g<2.92g×10-3g;
所以盐酸有剩余,溶液显无色。
考点: 化学方程式计算;数据的处理;酸碱指示剂的颜色变化

 全优冲刺100分系列答案
全优冲刺100分系列答案 英才点津系列答案
英才点津系列答案 红果子三级测试卷系列答案
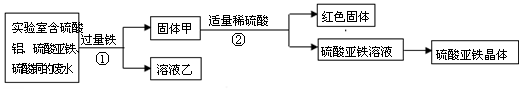
红果子三级测试卷系列答案取一定量Fe2O3与Al2O3的混合物,加入含溶质9.8g的稀硫酸,恰好完全反应.原混合物中氧元素的质量是( )
| A.0.8g | B.1.6g | C.3.2g | D.6.4g |
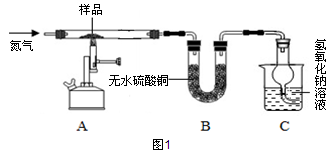
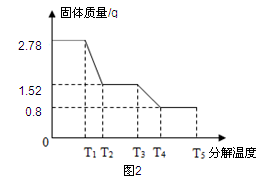
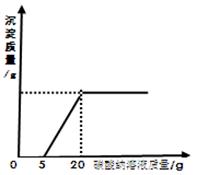
石灰石样品的主要成分是CaCO3(已知其它杂质不与盐酸反应).课外小组同学将50g盐酸分5次加入到20g该石灰石样品中,得到如下部分数据和图象:
| 次数 | 第1次 | 第2次 | 第3次 |
| 加入盐酸的质量/g | 10 | 10 | 10 |
| 剩余固体的质量/g | 16 | 12 | 8 |
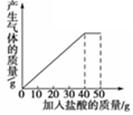
请计算:
(1)石灰石样品中杂质的质量为 g。
(2)所加盐酸的溶质质量分数.