��Ŀ����
����Ŀ�������д����л�ѧ��
��1���ú��ʵĻ�ѧ�������
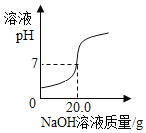
����Ȼ������Ҫ�ɷ�Ϊ_____���ڳ���Ǧ�������е�����_____��������������ȱ_____������Ͳ��������������ܲ�ͬ�ļ���Һ�������ƵĻ�ѧ���ʣ���������Һ�ж�������ͬ��_____��
��2���������ִ���������Ҫ�Ľ�ͨ���ߣ���ش��������⣺
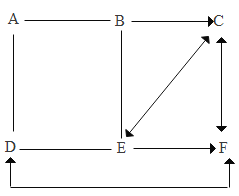
�ٲ���������_____ ����������ϡ����ϲ��ϡ��ϳɲ��ϡ�����������·�еĵ���һ��Ϊͭо�ߣ����������˽���ͭ��_____�ԡ�
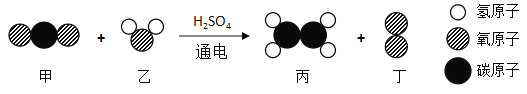
�ۼ�������ȼ��ͨ�������ͣ���������ʯ���и��ɷֵ�_____��ͬ������õ��ġ�
�������ʹ���ˡ�þ���Ͻ𡱣�����þԪ�ص�ij�����ӵĽṹʾ��ͼΪ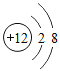 ��þԭ�ӵ�����������Ϊ_____��
��þԭ�ӵ�����������Ϊ_____��
���𰸡�CH4 H2SO4 Ca OH- ���ϲ��� ������ �е� 2
��������
��1���ú��ʵĻ�ѧ�������
����Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽΪ��CH4���ڳ���Ǧ�������е��������ᣬ��ѧʽ�ǣ�H2SO4���۸��������ں�����ߵĽ�����99%�����ڹ����������У�ʹ�ù��������ݾ��м�Ӳ�Ľṹ֧�ܣ�����������ȱCa������Ͳ��������������ܲ�ͬ�ļ���Һ�������ƵĻ�ѧ���ʣ���������Һ�ж�������ͬ�����������ӣ������ǣ�OH-��
��2���ٲ����������л��ϳɲ��Ϻ����ǽ��������Ƴɵģ����ڸ��ϲ��ϡ�������·�еĵ���һ��Ϊͭо�ߣ����������˽���ͭ�ĵ����ԡ�
�ۼ�������ȼ��ͨ�������ͣ���������ʯ���и��ɷֵķе㲻ͬ������õ��ġ�
�ܸ�������þԪ�ص�ij�����ӵĽṹʾ��ͼ��������֪��������������Ϊ12�������Ӻ�����10�����ӣ��ɼ���������þԭ��ʧȥ2�������γɵ�þ���ӣ����þԭ�ӵ�����������Ϊ2��