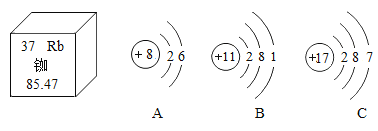
��Ŀ����
����Ŀ����ʵ���̼�������˵����������һ���߽���̼�������硣
(1)��д�йغ�̼���ʵĶ�Ӧ���ԡ�
������; | ���ʯ�и�� | ʯī���缫 |
��Ӧ���� | ��___________________ | ��______________ |
(2)��440��C��ѹ�����£��������������̼��Ӧ�����ɽ��ʯ(C) ��̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ____________��
(3)�� ̼����������֪����Ĺ�����ϣ���̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������(����ˮ)���������ʯ�ͼ������Կɻָ�ԭ״�����й���̼�����˵����ȷ����____________(����ĸ���)��
A ����������
B ���ظ�ʹ��
C �ɴ�������ʯ��й©.
(4)Һ̬������̼������������˾ȵ������Ϸ����Ļ��֣�����˵������ȷ����____________��
AҺ̬������̼��������Ⱦ��������
B ������̼�ɸ���ȼ������棬��������
C Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
���𰸡�Ӳ�ȴ� ���������� 3CO2+4Na![]() 2Na2CO3+C ABC AB
2Na2CO3+C ABC AB
��������
��1�����ʯ��Ӳ�ȴ����Կ����и����ʯī�������õĵ����ԣ����������缫�����Ӳ�ȴ��������ã�
��2��������̼������440���ѹ�����£���Ӧ���ɽ��ʯ��̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��3CO2+4Na![]() 2Na2CO3+C�����3CO2+4Na
2Na2CO3+C�����3CO2+4Na![]() 2Na2CO3+C��
2Na2CO3+C��
��3���ɶԡ�̼���ࡱ������֪������ж�ṹ�����Ժã�����ʯ�ͣ��ɴ�������ʯ��й©���к�ǿ���������������������ԣ����������ʯ�ͼ������Կɻָ�ԭ״�����ظ�ʹ�ã����ʴ�Ϊ��ABC��
��4��A��Һ̬������̼��������Ⱦ�������ϣ���A��ȷ��
B��������̼�ܶȱȿ����ɸ�����ȼ������棬�����������Ӷ����������ã���B��ȷ��
C��Һ̬������̼����ʱ���ȣ����ǿ�ȼ����Ż���Dz���ģ����ܽ��ͣ���C����
���AB��

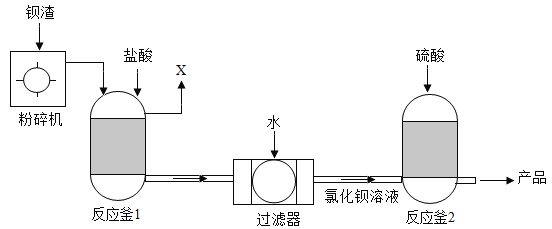
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�����Ŀ����֪20��CʱCa (OH) 2���ܽ��Ϊ0.165g,����20��Cʱ����7.4g���ʵı��ͳ���ʯ��ˮ�������:
(1)��������ʯ��ˮ������Ϊ____________g (��ȷ��1g)��
(2)����������ʯ��ˮ��ͨ��CO2,�����������ﵽ���ֵʱ��ͨ��CO2������Ϊ����?____ (д ���������)
(3)��֪: ![]() �� Ca (HCO3) 2 ������ˮ������ͼ�л���ͨ��CO2�����г��������ı仯����_____��
�� Ca (HCO3) 2 ������ˮ������ͼ�л���ͨ��CO2�����г��������ı仯����_____��
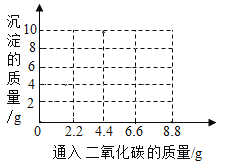
(4)��ȡһ�����ı��ͳ���ʯ��ˮ��ͨ��һ��ʱ���CO2,��Ӧ������������������±�
���� | Ca (OH) 2 | CO2 | CaCO3 | X | H2O |
����/g | 14.8 | 13.2 | 10 | a | 1.8 |
��a=______________����Ӧ�Ļ�ѧ����ʽΪ______________________________��