��Ŀ����
������ѩ������������һ��Сֽ��������д�š����������Ҫ�ɷ�����ʯ�ҡ�����1����ʯ��(CaO)����������������ǣ��û�ѧ����ʽ��ʾ��
________________________________
��2���Ҷ�������һϵ��̽������ý϶����棬��ʵ�鷽�����£�
������������������������� ʵ�鲽���������������������� ʵ�������������������������� ʵ�����
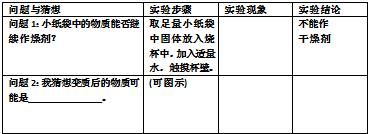
����1��Сֽ���е������ܷ������������� ȡ����Сֽ���й�������ձ��У���������ˮ���������� ���� �����������
����2���Ҳ�����ʺ�����ʿ�����
____________������������ ����ͼʾ������������������������������������������������������
��ÿС��ֻ��һ����ȷ�𰸣�
������
| ��1��CaO+H2O�T�TCa(OH)2
��2����������� ʵ�鲽�� ʵ������ ʵ����� ������ CaCO3����̼��ƣ� ȡСֽ���������������Թ��У�ע������ϡHCl�������ͼʾ��ʾ�� �����ݲ��� ��������̼��� �� ��2����������� ʵ�鲽�� ʵ������ ʵ����� ������ CaCO3����̼��ƣ� ȡСֽ���������������Թ��У�ע��ϡHCl�����Ѳ���������ͨ������ʯ��ˮ�У������ͼʾ��ʾ�� ��ϡHClʱ�����ݲ�����ʯ��ˮ����� ��������̼��ơ� �� ��2����������� ʵ�鲽�� ʵ������ ʵ����� ������ Ca(OH)2�����������ƣ� ȡСֽ���������������Թ��У����Թ��м���ˮ������ܽ��ȡ�ϲ���Һ������Һ�м���ɫ��̪�� ��̪��Һ����ɫ���ɫ ���������������� ���ʺ���������ж���̼�������?������ȫ������ʱ��?���ʱ�Ĺ����Ƿ�ȫ�����̼���?��������˵��������������Ҳ���֣������������뱾���йأ����ղ��÷֣����ر�˵���� ��2����������� ʵ�鲽�� ʵ������ ʵ����� ������ ��ش������ Ca(OH)2 ȡСֽ���������������Թ��У����Թ��м���ˮ������ܽ��ȡ�ϲ���Һ������Һ�м���ɫ��̪�� ��̪��Һ����ɫ���ɫ ������ʱ�ش��÷�̪���ļ���ܰ�ɫ�����е�Ca(OH)2��Ҳ�������ڼ�ˮʱ������CaO��ˮ��Ӧ���ɵ�Ca(OH)2��ɵģ��������Ƕ��߹�ͬ��ɵġ�ֻҪ���ǵ��д��CaO��ʵ��ĸ��Ų��Һ���
|
��ʾ��

 23�����ſ�ѧ��������ѧ�����ཡ���Ĺ�ϵԽ��Խ���У�ʳƷ���ʿ��Է�ֹʳƷ����������ʳƷ��Ӫ����ζ����ʳƷ���ʵĴ�ʩ�����ӷ��������������塢�Ÿ�������������ȣ�
23�����ſ�ѧ��������ѧ�����ཡ���Ĺ�ϵԽ��Խ���У�ʳƷ���ʿ��Է�ֹʳƷ����������ʳƷ��Ӫ����ζ����ʳƷ���ʵĴ�ʩ�����ӷ��������������塢�Ÿ�������������ȣ�