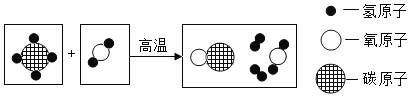
��Ŀ����
����Ŀ������������������������Ӧ�ù㷺��
��1�����ڽ���ͭ�������õ�__���������������ߡ�
��2������Ʒ�������ÿ���ʴ���ܵ�ԭ����_____��
��3������Ʒ��ʴ�Ĺ��̣�ʵ����������_____��_____�ȷ�����ѧ��Ӧ�Ĺ��̡���ҵ�ϳ���ϡ�����ȥ����Ʒ������⣬�÷�Ӧ�Ļ�ѧ����ʽ��_____��
��4��ij�����ŷŵķ�Һ�к����������������ᡣȡһ�����ķ�Һ��Ʒ��������������ۣ���ַ�Ӧ�����е�������_____����Ӧ��������Һ�������뷴Ӧǰ���_____����ѡ���������С�������жϡ�����
���𰸡������� ������һ�����ܵ�������Ĥ ���� ˮ Fe2O3+6HCl��2FeCl3+3H2O �������� ����
��������
��1�����ڽ���ͭ�������õĵ����Ժ���չ�ԣ��������������ߣ�
��2������Ʒ�������ÿ���ʴ���ܵ�ԭ�������Ļ�ѧ���ʱȽϻ��ã��ڳ�������������е�������Ӧ����������γ�һ�����ܵ���������Ĥ���Ӷ���ֹ����һ������ʴ��
��3������Ʒ��ʴ�Ĺ��̣�ʵ����������������ˮ�ȷ�����ѧ��Ӧ�Ĺ��̣�
�������Ҫ�ɷ�����������������������������ϡ���ᷴӦ�����Ȼ�����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��![]() ��
��
��4�����Һ��Ʒ�У�������������ۣ��������ᷴӦ���������������������ʳ�ַ�Ӧ�����е������ǣ�����������
�������ᷴӦ��![]() ��56�����û���2���������ʷ�Ӧ��������Һ�������뷴Ӧǰ�������
��56�����û���2���������ʷ�Ӧ��������Һ�������뷴Ӧǰ�������

 ȫ��������ϵ�д�
ȫ��������ϵ�д� һ��һ����ʱ���ϵ�д�
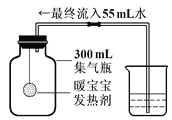
һ��һ����ʱ���ϵ�д�����Ŀ����������������治���������տ����еĶ�����̼������[NaOH(Na2CO3)]��ij��ѧ����С���ͬѧ��Ϊ�˲ⶨʵ�����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ��(ͼ������̨�Ѿ���ȥ)��ʵ����27�桢101kPa�½��С�
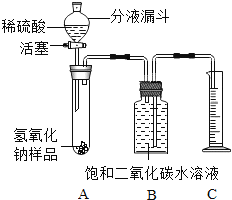
ʵ�鲽������:
�ٰ�ͼ���Ӻ�װ��;
������ƽȷ��ȡ����������Ʒmg,����A���Թ��ڣ���B�м���ƿ�ڵ��뱥�Ͷ�����̼ˮ��Һ��ƿ����;
�����Һ©���е���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ������Ͷ�����̼ˮ��ҺVmL;
�ܼ�������������Ʒ��̼���Ƶ�����������
��ش���������:
��1���������Ʒ������ʵĻ�ѧ����ʽ:________��
��2����ʵ�鲽��ٺ͢�֮�䣬��ȱ��һʵ�鲽�裬��ʵ�鲽����_______��
��3��B�м���ƿʢװ�ı��Ͷ�����̼ˮ��Һ������ˮ���棬��ԭ����_______��
��4�����������������Ʋ��ֱ��ʵķ���:
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ��������ˮ���μӹ�����_______ | ������ɫ���� | �������Ʋ��ֱ��� |
�ھ��ã����ϲ���Һ�еμ�________ | _____ |
��5��ȡ10g���ʵ������������ձ��У�����̼Ԫ�ص���������Ϊ6%�����ձ��м���100gһ������������ϡ����(����)����Ӧ�������ձ������ʵ���������____g