��Ŀ����
����Ŀ�����ͼʾʵ��װ�ã��ش��������⡣
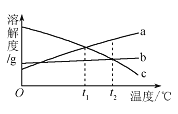
��1��ͼ��a��b���������ƣ�a__________��b__________��
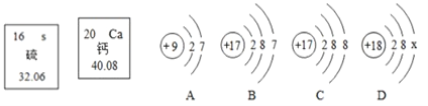
��2���ø�����ع�����������ѡ�õķ���װ����________������A������B������C��������Ӧ�Ļ�ѧ����ʽΪ_________________��������Dװ���ռ�O2���������ռ����ʱ��Ӧ��________��������ˮ��ȡ��������������Ϩ��ƾ�������������Fװ���ռ�O2���������ķ�����________________________________��
��3��ʵ������ȡ����ʱ������Gװ���ռ�H2��������Ӧ��______��ͨ�루����c������d������
��4��С��ͬѧ��̽��ʵ��������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼���Һ�����ʵijɷ֣�����һͬ����̽�����ش����⡣
��������⣩��Һ�е�������ʲô���ʣ�
���������룩С����Ϊ��Һ������ֻ��CaCl2������Ϊ�����ܺ��е�������_________���ѧʽ����
���������ϣ�CaCl2��Һ�����ԡ�
��ʵ������ۣ�������д��̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ______________________________��
���ֱ�ȡ�����÷�Һ��CaCl2��Һ���뵽��֧�Թ��У��������зֱ������ɫ��̪��Һ���Ա�ʵ�飬�����֧�Թ�����Һ����ɫ������С����Ϊ�Լ��IJ�������ȷ�ġ�
������ΪС���ʵ�� ______����������������������֤�����IJ��룬������ ___________________��
�����Ҫ֤����IJ�������ȷ�ģ������ѡ��_________������ĸ���������̪��Һ��
A ʯ����Һ B ����������Һ C ϡ���� D ��������Һ
����չ��Ӧ�ã�����ʵ��֤������IJ�������ȷ�ģ����Һ�м�������ģ���Ӧ��ɺ���ˣ����ɵõ�ֻ��CaCl2һ�����ʵ���Һ��
�ھ�ʵ��֤������IJ�������ȷ��ijͬѧ������������ʵ�飺ȡ��Һ20.0g���ձ��У���ε���̼������Һ������������̼������Һ������/g�������ɳ��������� (/g)�ı仯��ϵ��ͼ��ʾ���Լ��㣺

��������Һ��CaCl2������������___________
������̼������Һ�����ʵ�����������___________��
���𰸡��Թ� ����ƿ A 2KMnO4�� K2MnO4+MnO2+O2�� ��ˮ��ȡ�������� �������ǵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ����ȼ��֤���������� d HCl CaCO3 + 2HCl == CaCl2 + H2O + CO2�� ���� HCl�Ĵ���Ҳ����ʹ��̪��Һ��ɫ A 5.55% 5.3%
��������
��1���Թ��dz��õķ�Ӧ����������ƿ���ռ�������������ʴ�Ϊ���Թܣ�����ƿ��
��2������ø����������������Ҫ���ȣ�ѡ�õķ���װ����A������������ȷֽ���������غͶ������̺�������Ҫע����ƽ��ʵ�����Ӧ���Ƴ����ܣ���Ϩ��ƾ��ƣ���ԭ���ǣ���ֹˮ������ʹ�Թ�ը�ѣ����������������ǣ��������ǵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ����ȼ��֤���������ˣ��ʴ�Ϊ��A��2KMnO4�� K2MnO4+MnO2+O2������ˮ��ȡ�������ܣ��������ǵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ����ȼ��֤���������ˣ�
��3��ʵ������ȡ����ʱ������Gװ���ռ�H2��������Ӧ�Ӷ̹ܽ��룬��Ϊ�������ܶȱȿ���С���ʴ�Ϊ��d��
��4��С����Ϊ��Һ������ֻ��CaCl2������Ϊ�������ʱ�����������Ȼ��ƺ�����Ļ���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����ƽ���ɣ�����ΪС���ʵ�鲻��֤�����IJ��룬������HCl�Ĵ���Ҳ����ʹ��̪��Һ��ɫ��Ҫ֤�������ᣬ���Եμ���ɫʯ����Һ����ɫ���ɫ����֤��������ʴ�Ϊ��HCl��CaCO3 + 2HCl == CaCl2 + H2O + CO2�������ܣ�HCl�Ĵ���Ҳ����ʹ��̪��Һ��ɫ��A��
����չ��Ӧ�á���ʵ��֤�����ҵIJ�������ȷ�ģ����Һ�м��������̼��ƣ���Ӧ��ɺ���ˣ����ɵõ�ֻ��CaCl2һ�����ʵ���Һ���ʴ�Ϊ����̼��ƣ�
�����Һ���Ȼ��Ƶ�����Ϊx���μӷ�Ӧ��̼���Ƶ�����Ϊy��

x=1.11g y=1.06g��
�÷�Һ��CaCl2����������Ϊ��![]()
̼������Һ�����ʵ���������Ϊ��![]()
�𣺣������÷�Һ��CaCl2����������Ϊ5.55%
������̼������Һ�����ʵ���������Ϊ5.3%

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ�ⶨij��Ʒ��̼���ƺ��Ȼ��ƵĻ�����̼���Ƶ�����������ij��ѧ��ȤС���������ʵ�飬ȡ50g����Ʒ�������ձ��У�Ȼ��ȡһ����������������ϡ����200g�����ĵȷ֣�ÿ����װ��Ʒ���ձ��м���50g��ʵ���ü���ϡ����������뷴Ӧ���ձ������ʵ��������Ĺ�ϵ���±�����
����ϡ���������/g | 50 | 100 | 150 | 200 |
��Ӧ���ձ������ʵ�������/g | 91.2 | 132.4 | 182.4 | m |
��1������m��ֵΪ________��
��2����Ʒ��̼���Ƶ�����������________����ȷ��0.1%����
��3������ϡ�����������������________��д������ļ�����̣���