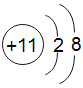��Ŀ����
����Ŀ������Ǽס������ֹ���(�����ᾧˮ)�ڲ�ͬ�¶��µ��ܽ��(��λ:g/100gˮ)
�¶�(��C) | 0 | 20 | 40 | 60 | 80 | 90 | 100 |
�� | 13.3 | 32 | 64 | 110 | 169 | 202 | 246 |
�� | 35.7 | 36 | 36.6 | 37.3 | 38.4 | 39 | 39.8 |
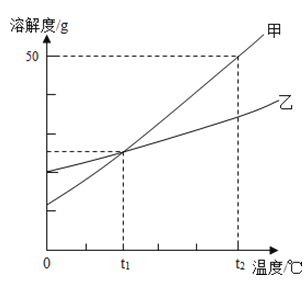
��1��60��Cʱ���ܽ��Ϊ______����60g����50gˮ�г���ܽ�õ���Һ________g��
��2�����ҵ���Һ����ȡ�ҵķ�����_______(ѡ�������½ᾧ�����������ᾧ��)��
��3���ֱ�100g�ס��ҵı�����Һ��60��C������20��C����������Һ��������ȷ����______
A.�ס��Ҿ�Ϊ������Һ
B.��Һ��������>��
C.�����ܼ���������<��
D.����������������>��
���𰸡�110g 105 �����ᾧ AC
��������
��1��������Ŀ��������ҵ�60���£����ܽ��Ϊ110g��100gˮ������60�����ܽ�110g�ļף���50gˮ�ڸ��¶��£�ֻ���ܽ�һ�����������50gˮ��60�����ܽ�55g�ף�������Һ����=ˮ������+�ܽ�ļ�����=50g+55g=105g��
��2�������ҵ��ܽ�����ݣ���֪���ҵ��ܽ�����¶ȱ仯�������¶Ȳ����ھ��������������ܼ�����ʹ�������������Բ��������ᾧ�ķ�����ȡ�ң�
��3��A���ס��ҵ��ܽ�����¶Ƚ��Ͷ����ͣ����Լס��ҵı�����Һ��60�潵����20��ʱ�������о�����������ʱ�Զ��DZ�����Һ��ѡ��A��ȷ��
B�� �����ܽ�ȹ�ʽ![]() ����֪60��ʱ��100g�ס��ұ�����Һ�У�����������Ϊ52.4g���ܼ�����Ϊ100g-52.4g=47.6g������������Ϊ27.2g���ܼ�����Ϊ100g-27.2g=72.8g����������20��ʱ���ܼ��������䣬�����ܽ�ȹ�ʽ
����֪60��ʱ��100g�ס��ұ�����Һ�У�����������Ϊ52.4g���ܼ�����Ϊ100g-52.4g=47.6g������������Ϊ27.2g���ܼ�����Ϊ100g-27.2g=72.8g����������20��ʱ���ܼ��������䣬�����ܽ�ȹ�ʽ![]() ��������ü���Һ�У�������15.2g������Һ�У��ҵ���������26.2g����ʱ����Һ����=47.6g+15.2g=62.8g������Һ����=72.8g+26.2g=99g�����Լף��ң�ѡ��B����
��������ü���Һ�У�������15.2g������Һ�У��ҵ���������26.2g����ʱ����Һ����=47.6g+15.2g=62.8g������Һ����=72.8g+26.2g=99g�����Լף��ң�ѡ��B����
C������B���㣬��֪���ܼ�����Ϊ47.6g���ҵ���������Ϊ72.8g�����Լף��ң�ѡ��C��ȷ��
D����ʱ��Ϊ������Һ���ܽ��Խ�������ܽ�Խ�࣬��������Խ��20���¼��ܽ��С���ң�����������������Ϊ�ף��ң�ѡ��D����ѡAC��

����Ŀ����һ���ܱ����������������ʣ���һ����������ȫ��Ӧ��÷�Ӧǰ������ʵ��������£�
���� | X | Y | Z | Q |
��Ӧǰ����/g | 4 | 10 | 1 | 21 |
��Ӧ������/g | 0 | 12 | 15 | ���� |
��֪X����Է�������ΪN��Q����Է�������Ϊ2N������������ȷ����( )
A. ��Ӧ��Q������Ϊ9 g
B. ��Ӧ��Y��Q�����ı������֮��Ϊ1��1
C. ��Ӧ������15 g Z
D. X��Q��Ӧǰ�Ļ�ѧ������֮��Ϊ2��1
����Ŀ��С����ͼA��ʾװ�öԶ�����̼�����ʵ�����̽�����۲쵽��������_____��ʵ��Ľ�����_____
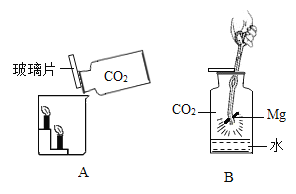
��������⣩������̼�ܷ�֧��ȼ�գ�
��������裩þ���ڶ�����̼��ȼ�ա�
���������ϣ�������þ��������þ���ǰ�ɫ������ˮ�Ĺ��塣
��MgO��2HCl=MgCl2��H2O
��MgCl2��2NaOH=Mg��OH��2����2NaCl
��ʵ��̽������ͼ��þ������ȼ�գ�ð���̣��к�ɫ�������ɣ����ų��������ȡ�
��Ϊ����������ijɷ֣�������ʵ�顣
ʵ�鲽�� | ʵ������ | ʵ����ۺͻ�ѧ����ʽ |
�����������Ĺ��ƿ�м������ϡ���ᣬ��ַ�Ӧ����ˣ�����ֽ�����к�ɫ���� | ||
��������ɫ�����ռ���ϴ�ӡ������ȼ���ڻ����Ϸ���һ��պ�г���ʯ��ˮ���ձ� | a����ɫ����ȼ�գ��ձ��ڱڳ��ְ�ɫ���� | b����ɫ������_____����Ӧ�Ļ�ѧ����ʽ�ǣ� _____��_____ |
����ȡ������Һ���Թ��У���μ�������������Һ | c����ʼ_____�����а�ɫ�������� | d�����̵ijɷ���_____ |
����˼��ߣ�ʵ������ijЩ���ý���������Ż𣬲����ö�����̼���Ӧ��ϸɳ���