��Ŀ����
����Ŀ�������д����л�ѧ����ѧ������������أ�
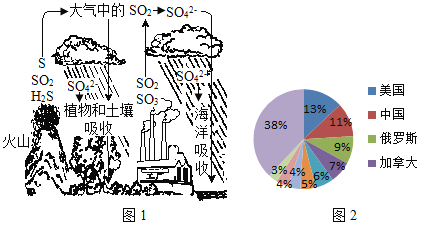
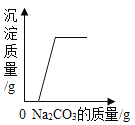
��1�����������������������ڣ���Ҫ��ȡ�϶�ĵ����ʣ���ͼ�е����ʺ�����ߵ�____��
A ���� B ������ C �� D ����
��2���ӵ�������������ָ��____������Ԫ������������������������ȱ����ܻ��еļ�����____��������״���״���������������������
��3�����dz���ϴ�Ӽ���ϴ�;��ϵ����ۣ�������Ϊϴ�Ӽ�����____���ܣ���������������ˮ����������____������ʳ��������ʳ��ˮ������
��4�������е�������Ʒ��ʹ�õ���Ҫ�������ںϳɲ��ϵ���____������ţ���
�� ����ִ��� ��
����ִ��� �� �ȶ�������
�ȶ�������  ����Χȹ ��
����Χȹ �� ���ϱ���Ĥ
���ϱ���Ĥ
��5��������IJ˵�ʹ�ú�ȡ�Ĵ�ʩ����ֹ����____��
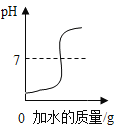
��6�������е�����������ˮ�в����γ���Һ����____��
A ʳ�õ����� B ʳ�� C ��� D ζ����
���𰸡�C Ԫ�� ��״���״� �黯 ʳ�� �ڢ� ���� AC
��������
��1��A����������ˮ�������к��зḻ��ά���أ���A����ȷ��
B�������������߲ˣ����к��зḻ��ά���أ���B����ȷ��
C�����к��зḻ�ĵ����ʣ���C��ȷ��
D�������к��зḻ�����࣬��D����ȷ����ѡC��
��2���ӵ����е������������Ե��ʡ����ӡ�ԭ�ӵ���ʽ���ڣ�������ָ����������ǿ�����ڵ�Ԫ�أ��������̬�أ��ʼӵ�������������ָ��Ԫ�أ�������ȱ����ܻ��еļ����Ǽ�״���״�
��3��ϴ�Ӽ������黯�����ܰѴ���͵α������СҺ�Σ����黯���ã�����ϴ�Ӽ���ϴ�;��ϵ������������黯���ܣ�ˮ������Ҫ�ɷ���̼��ơ�������þ��ʳ���к��д��ᣬ���������ԣ��ܺ�̼��ơ�������þ��Ӧ���Ӷ���ˮ����ȥ���ʳ�������������ˮ����������ʳ�ס�
��4���ٲ���ִ������ڽ������ϣ��ʲ���ȷ��
���������õ��Ǻϳ������ںϳɲ��ϣ�����ȷ��
����Χȹ��������ά�Ƶģ��ʲ���ȷ��
�����ϱ���Ĥ���������Ƴɵģ�������������ϳɲ���֮һ������ȷ����ѡ�ڢܡ�
��5��������IJ˵�ʹ�ú�ȡ���ɵĴ�ʩ����ֹ���⡣
��6��A��ʳ�õ����Ͳ�����ˮ����ˮ������γ�����Һ����A����ȷ��
B��ʳ��������ˮ����ˮ��Ͽ��γ���Һ����B��ȷ��
C����۲�����ˮ����ˮ������γ�����Һ����C����ȷ��
D��ζ�����ܽ���ˮ����ˮ��Ͽ��γ���Һ����D��ȷ����ѡAC��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�����Ŀ����ѧ���ϸı������ǵ�����������ø����á�
��1����ͼ�е�������Ʒ����Ҫ���л��ϳɲ����Ƴɵ���___________(����)��

��2��ѧ�û�ѧ���������Ǿ���Ӫ���������ijƷ�����ʰ��ӵ����ϱ�����������и�����Ӫ������______________��Ϻ�ʡ����⡢�����и�����Ӫ������__________��
���ϱ� | |
����Ƥ | ��ۡ������� |
���� | Ϻ�ʡ����⡢������ �ײˡ������͵� |
��3�������е�������Ʒ�ڲ��ϵظ��»�����
������PPR����ˮ������ȡ����������ˮ�ܡ���Ϊ��������ˮ������_____��_____���ö���ʴ��
��Һ�������������Ϊ�������������������������ԭ����_____________�����۽Ƕȷ���������ѹ����Һ����ԭ����______________��
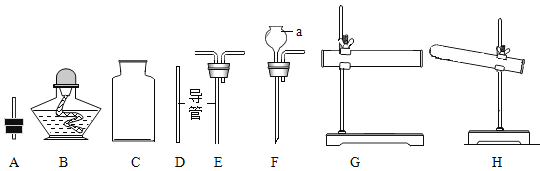
��4����Щ������С��ͬѧ���г���ȼ�ϵ��ݱ������ͼ��ʾ��

�ٴӻ����ĽǶȿ�����ú����ú��Ƚϣ���Ȼ�����ŵ���_______________��
��д��һ������ȼ��ȼ�յĻ�ѧ����ʽ_____________��
��5������ʹ�û�ѧ���ʡ����û�ѧԭ�����Խ�������������⡣
�ټ��þ�ˮ�������������õ�������_______________�������п���____________����Ӳˮ����ˮ��
�ڳ���ʱ�����е������Ż���õ������_______________��
��84����Һ����NaClO�������飨��HCl�����ܻ��ã���Ϊ���ǻᷴӦ����һ���ж�����X����Ӧ�Ļ�ѧ����ʽ��NaClO+2HCl=NaCl+H2O+X����X�Ļ�ѧʽ��_______��
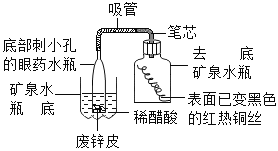
����Ŀ��Сǿͬѧǰ�����ص�ʯ��ʯ�������е��飬��ȡ�������ɿ��ʯ��Ʒ������Ʒ��̼��Ƶ������������м�⣬���õİ취���£�ȡ��8 g����ʯ��ʯ��Ʒ����40 gϡ�����4�μ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�к������ʲ�����ˮ���������ᷴӦ��������㣺
��1��8 gʯ��ʯ��Ʒ�к������ʶ��ٿ�____________��
��2����Ʒ��̼��Ƶ����������Ƕ���_________________��
��3���±���m����ֵӦ��Ϊ����____________��
��� | ����ϡ��������/g | ʣ���������/g |
��1�� | 10 | 5.5 |
��2�� | 10 | m |
��3�� | 10 | 1.2 |
��4�� | 10 | 1.2 |
��4��Ҫ�õ�280 kg CaO����Ҫ��������Ϊ80%��ʯ��ʯ����ǧ��_____________��(��ѧ����ʽ��CaCO3![]() CaO+CO2��)
CaO+CO2��)
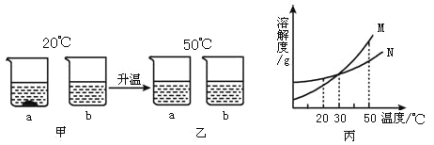
����Ŀ���Ӻ�ˮ�õ��Ĵ����������п��������ʣ��Ȼ�þ���Ȼ��Ƶȣ��Ͳ��������ʣ���ɳ�ȣ���������з�����ᴿ��������ڹ�ҵ���������ǵ��ճ����ʵ����ģ�ҵ�����ᴿ��������ͼ����ش�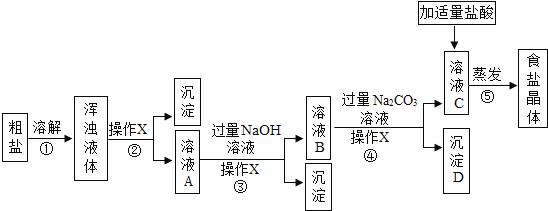
��1������ٺ͢ڵ�Ŀ����________������۵���ҪĿ����________��
��2������X��������________���ò������õ��IJ����������ձ�����������________������ҺC�м����������ᣬ�������������IJ���������________��
��3����ʵ�����ҺC�õ����Σ����õ��������ܼ��ķ��������ý�����Һ�¶ȵķ������ο��ܽ�����ݷ���ԭ��________��
��4���±��ṩ���������ʵ��ܽ�����ݣ���ȡ��Ϣ��ش�
�¶ȡ� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ��/g | NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
KNO3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |

��ͼ�жϣ��Ȼ��Ƶ��ܽ����������ͼ________�������ֱ�ţ���40��ʱ����60g���������100gˮ�У�������10��ʱ����������ؾ���________ g��