��Ŀ����
��̼����������ͨ��һ���ķ���������ҵ�����в����Ķ�����̼����������������ã�ijУ����С���ͬѧ���������������Һ��������������̼��������������£���������������δ�������

��ش������й����⣺
��1�������ҡ��з�����Ӧ�Ļ�ѧ����ʽΪ���� ����
��2�������������������У�����ѭ�����õ��������� ����
��3�������й������д�������� ��������ţ���
| A���ü�������Ч����̼�ŷ� |
| B������Ӧ���롱�����У��������ʵĻ�������ʱ�����ᾧ���� |
| C���ܺĴ��Ǹü�����һ��ȱ�� |
| D�����������Ķ�����̼�������������ء��״���̼����狀ʹ���� |
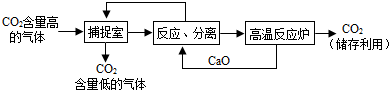
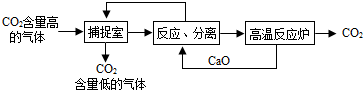
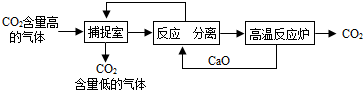
�����������1�������к�������������Һ�������������������̼��Ӧ����̼���ƺ�ˮ�����2NaOH+CO2=Na2CO3+H2O��
��2����ѭ���Ĺ����У������ƺ��������ƶ�����ѭ�����ã���������ơ��������ƣ�
��3��A��������������Һ��������������̼�����Լ��ٶ�����̼���ŷţ��Ӷ�����̼�ŷţ���A��ȷ��
B��������������̼���Ʒ�Ӧ����̼��Ƴ������������ƣ����Բ��ù��˵ķ������룬����Ҫ���������ᾧ�IJ�������B����
C���ù����У���Ҫ���£��ܺĽϴ�C��ȷ��
D��������̼����Ҫ�Ļ���ԭ�ϣ��ʡ��������Ķ�����̼�������������ء��״���̼����狀ʹ����D��ȷ��
����B��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�