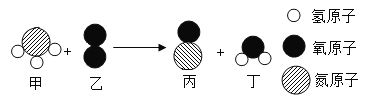
��Ŀ����
����Ŀ���������У���Ԫ������Ҫ���������ܣ�����ˮ����ά�����ƽ�⡢ά��Ѫѹ�ȡ��ҹ�������ʳ��ÿ��ͨ��ʳƷ����ϻ����Ԫ�ص���Ҫ��Դ��ͼ��ʾ��
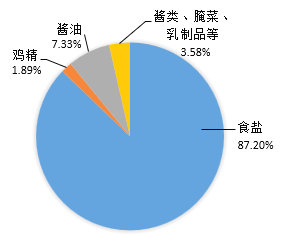
��ͼΪ2015���ҹ�ijʡ���˾�ÿ��ʳ��������������Ƽ���������������֯��WHO���Ƽ��Ĺ��ʱ��ıȶԡ�
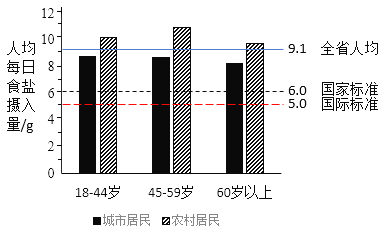
��ʵ֤�����й��˵���ʳ�ṹ����Ԫ�ع��࣬��Ԫ�ع��٣�������������¸�Ѫѹ�ķ�����Ϊ�ˣ��Ե���Ϊ��������������ζ���Ȼ��أ����Ȼ��ش��沿���Ȼ��ƣ��������أ���ʹ�ơ��ر����������γɵ����Ρ������������ڷ��θ�Ѫѹ��ͨ�������Ƽ����Ρ���������ҵ�ߡ��������Ͷ�ǿ�ȵĹ�����Ա����Һ��ʧ����ߴ��������ӣ������ಡ���Ѫ֢���߲�����ѡ�á����������г���������κ͵������γɷ����ϱ�
���� | NaCl ��g/100 g�� | KCl ��g/100 g�� | KIO3����I�ƣ���mg/kg�� | K4Fe(CN)6���ԣ�Fe(CN)6��4-�ƣ���mg/kg�� |
����� | ��98.5 | 0 | 18~33 | ��10 |
�������� | 65~80 | 20~35 | 18~33 | ��10 |
�����������ݻش��������⡣
��1����ͼ��֪�����ǻ����Ԫ�ص���Ҫ��Դ��_____��
��2��ʳ����K4Fe(CN)6����Ҫ�����ǿ������K4Fe(CN)6����_____��Ԫ����ɵġ�
��3����ͼ��֪������˵������ȷ����_____������ĸ��ţ���
A ��ʡ���˾�ÿ������ʳ�ε��������˹����Ƽ��ı�
B �ҹ���ʳ�����������Ƽ�����������������֯��WHO���Ƽ��Ĺ��ʱ�
C �����dz��о�����ũ�����������Խ���˾�ÿ������ʳ��Խ��
D ��ͼʾ��ÿ��������У�ũ�������˾�ÿ��ʳ�����������ȳ��о����
��4�������μȱ�֤�˵�ζ���������ڷ��θ�Ѫѹ��ԭ����_____��
��5�����ݱ����㽨������С�������ȵ�����ѡ�õ�����_____��
���𰸡�ʳ�� �� C �����Ȼ��ش��沿���Ȼ��ƣ��Ȼ�������ζ���ҿɴﵽ�������ص�Ч�� �����
��������
��1�����ҹ�������ʳ��ÿ��ͨ��ʳƷ����ϻ����Ԫ�ص���Ҫ��Դͼ��֪��87.20%��Դ��ʳ�Σ�7.33%��Դ�ڽ��ͣ�3.58%��Դ�ڽ��ࡢ��ˡ�����Ʒ�ȣ�1.89%��Դ��ζ���������Ԫ�ص���Ҫ��Դ��ʳ�Σ����ʳ�Ρ�
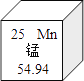
��2��K4Fe(CN)6�ɼ�Ԫ�ء���Ԫ�ء�̼Ԫ�ء���Ԫ�ع�����Ԫ����ɣ�����ġ�
��3��A���Ӹ�ʡ��2015���˾�ÿ��ʳ��������������Ƽ���������������֯�Ƽ��Ĺ��ʱ��ıȶ�ͼ���Կ���ȫʡ�˾�ÿ��ʳ����������9.1g�����������Ƽ��ı�6.0g��ѡ��A���������⣻
B����ͼ�п�֪�ҹ��Ƽ��ı����˾�ÿ��ʳ��������6.0g������������֯�Ƽ��Ĺ��ʱ����˾�ÿ��ʳ����������5.0g������ҹ���ʳ�����������Ƽ�����������������֯��WHO���Ƽ��Ĺ��ʱ���ѡ��B���������⣻
C����ͼ�п�֪��ũ�����45-49����˾�ÿ������������18-44��Σ���˲�������Խ��������Խ�٣�ѡ��C�������⣻
D����ͼ�пɿ���ͼʾ��ÿ��������У�ũ�������˾�ÿ��ʳ�������������ڳ��о���ѡ��D���������⡣��ѡ��C��
��4���й��˵���ʳ�ṹ����Ԫ�ع��࣬��Ԫ�ع��٣�������������¸�Ѫѹ�ķ������������м�������ζ���Ȼ��أ����Ȼ��ش��沿���Ȼ��ƣ�ʹ�ơ��ر��������������ڷ��θ�Ѫѹ����������Ȼ��ش��沿���Ȼ��ƣ��Ȼ�������ζ���ҿɴﵽ�������ص�Ч����
��5������С�������ȵ����칤�������ڸ�����ҵ�ߣ���Һ��ʧ����ߴ��������ӣ���Ҫ���������ӣ����Ӧѡ������Σ��������Ρ�

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�����Ŀ�����A��D����ѡ��������
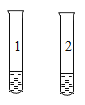
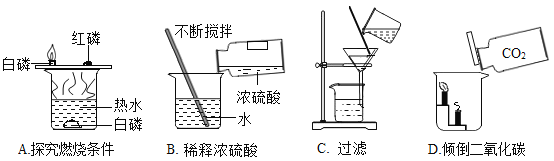
��ͼ��ʾ������֧�Թ��н���ʵ�飬��ȫʵ�鷽����
��� | Ŀ�� | ���� |
A | ��֤_____ | ���Թ�1�м���һС�����5mL80����ˮ���Թ�2�м���һС�����5mL��ˮ���ڷֱ�ͨ������ |
B | ����ϡ�����̼������Һ | ���Թ�1��2�зֱ�������ִ�����Һ���ڷֱ����_____��Һ |
C | �Ƚ�����ͭ�����Ľ������ | ���Թ�1�м���FeSO4��Һ���Թ�2�м���AgNO3��Һ�� �ڷֱ����_____ |
D | �Ƚ�_____ | ���Թ�1�м���һ��������غ�5mL���ͣ��Թ�2�м���һ�����5mL���ͣ����� |
����Ŀ�����A��B��������ѡ1����������������𣬰�A�Ʒ֡�ͼ��װ�õļг���������ȥ��
A����ͼװ���о����� | B����ͼװ���о�������̼ |
|
|
��1���������������Һ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ____�� ��2��ľ̿��������ȼ��ʱ��������____�� | ��1������ϡ�������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_____�� ��2��ʯ����ֽ����������_____�� |
����Ŀ����������ȤС��ͬѧ�Ա�¶�ڿ������������ƹ����̽������ش��������⣺
��������⣩
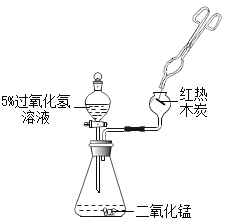
���������ƹ�����û�б��ʣ�
������ʵ�飩
��ͬѧȡ�����������Թ�����������ˮ�ܽ⣬������������Ȼ�����Һ���۲쵽__________��֤���������ƹ����Ѿ�����̼���ơ�
��������⣩
��γ�ȥ�������ƹ����е����ʣ��õ����������������أ�
������ʵ�飩
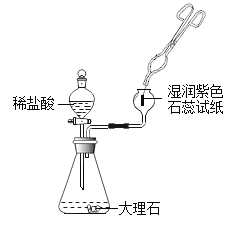
��ͬѧ�Ը��������ƹ�������ᴿ�������²������̽���ʵ�飺

��ʵ�������
��1������ڵ���Ҫ����������__________��������еķ�Ӧ�Ļ�ѧ����ʽ��__________��
��2��������м����Թ�������������Ŀ����__________��
��3��Ϊ��ô������������ƣ�������������ľ������������Ũ����__________��ѡ���������ᾧ���������½ᾧ���������ˡ�
��4����ͬѧ��Ϊ��ͬѧ�����е�������������ˮ����Ҫʹ�ýϴ�����ˮ����������Ѷȣ�������_______�Լ����档
��ʵ����չ��
��βⶨ���õ��ռ���Ʒ���������Ƶ�����������
��һƿ���õ��ռ��г�ȡ20g�������ʣ������������Ϊ19g����ȫ����ˮ�����100g��Ʒ��Һ����ȡһ�����ʵ������������Ȼ�����Һ����Ʒ��Һ��ϣ���ַ�Ӧ��õ������ʾ�����ݡ�
��Ŀ�ʹ��� | ��1�� | ��2�� | ��3�� | ��4�� |
��Ʒ��Һ������g�� | 10 | 20 | 30 | 40 |
�Ȼ�����Һ������g�� | 10 | 15 | 15 | 30 |
����������������g�� | 1.97 | 3.94 | 3.94 | X |
���е�__________�η�Ӧǡ����ȫ����
��5���������Ʒ���������Ƶ�����������__________����д���������̣�