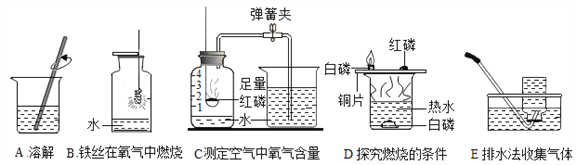
��Ŀ����
����Ŀ���Ķ�������ն��ģ��������������¸�д����
�ɻ����ֳ�Ƥ�����䵰���Ұ����ȣ���������Ǻ�״�ĵ����г�������״�Ľᾧ���ƶ���������һ���ҹ���ͳ��ζʳƷ���ڸ��ʻ�ˬ�ڣ�ɫ��ζ���ж���֮������ҽ��Ϊ���ɻ�������������ʹ����ʹ�ȼ�����һ����Ч�������ɻ�������һ�ɼ�ɬζ���ڳ��ɻ�����ʱ����Լ��������Ľ���֭��
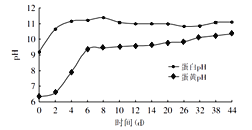
�ɻ��������������Ѽ���������ȼӹ����ɡ�����������ʯ�ҡ����ʳ�Ρ���衢��ľ�ң���Ҫ�ɷ�K2CO3����ԭ����ˮ��������Ͼ����Ƶá��������������У�ͨ��һϵ�з�Ӧ��������ǿ�NaOH��KOH�����������뵽����͵����У������еĵ��������ã���ʹ�����ʷֽ⡢���̲��ų�������������Ͱ�����ͬʱ����ļ���뵰���ʷֽ���İ������һ�������кͷ�Ӧ�����ɵ��εľ������������̬�ĵ����У�������˶�䡰�ɻ������������������뵰��͵����еĿ������������ɸ������ʹ����͵��Ƶ���ɫ�����ı䣬���������IJ��ɫ���������ī��ɫ����ͼ��ʾ�ɻ�������ʱ�������е���͵��Ƶ�pH�ı仯��ϵ��
�ܶ�����ƽʱ�����ж��dz�ϲ����Ƥ�������ࡢ�����ɻ�����ר����������ʳ���ٳ�����ȴ���ܹ���ʳ�á���һ���ɻ������ƹ����е����ʷֽ⡢���ʣ�����Ӫ����ֵ���һ�����ƻ�������������ɻ��������ܺ�Ǧ�����ҹ涨�ɻ�����Ǧ������С��0.5mg/kg������Ǧ�����ױ���ͯ���գ�����Ǧ�ж�������������Ƥ���ɻ���1��2Сʱ��һ��Ҫ���꣬����ʱ�䱩¶�ڿ����У��dz�����Ⱦɳ���ϸ˾���ɳ���ϸ˾�����ٷ�ֳ����ʱʳ���ɻ��������������ж�����,�����������ݣ��ش��������⡣
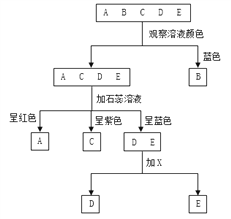
��1��ʳ���ɻ���ʱ������������֭���Գ�ȥ�ɻ�����________ζ��
��2���������������л�����ǿ��NaOH��KOH����Ԫ��������ԭ���е�________����д��Na2CO3��Ca(OH)2��Ӧ�Ļ�ѧ����ʽΪ________��
��3�������ɻ�������ʱ�������е���͵���pH�ı仯��ϵ�жϣ�������͵���pH���ﵽ9����ʱ���ɻ����������Ƶ�����Ϊ________������ĸ��ţ���ͬ����
A��2�� B��6�� C��12�� D��32��
��4�����й����ɻ�����˵����ȷ����________��
A���ɻ���������ɫ���γ������������й�
B���ɻ������кܸߵ�Ӫ����ֵ�����˿��Զ��
C�������ɻ������ܺ���������Ǧ����ͯ����ʳ��
D������Ƥ���ɻ�����ҹ���ú���Ȼ���Է���ʳ��
���𰸡� ��ɬζ ��ľ�ң���K2CO3�� Ca(OH)2 + Na2CO3 === CaCO3��+ 2NaOH B AC
�������������ڡ��ɻ��������龳�¿����εĻ�ѧ���ʣ���ʯ�ҵ���������;�������غ㶨�ɼ�������Ϣ��������Ϣ�������������Ķ���������е�֪ʶ���з�����
��1������֭�������������кͼ�ˮ��ʳ���ɻ���ʱ������������֭���Գ�ȥ�ɻ����м�ɬζ��
��2���������������л�����ǿ��NaOH��KOH����Ԫ��������ԭ���еIJ�ľ�ң���K2CO3������ʯ������ˮ��Ӧ�����������ƣ����ɵ�������������̼���Ʒ�Ӧ���ɲ�����ˮ��̼��ƺ��������ƣ���Ӧ�Ļ�ѧ����ʽΪ��Ca(OH)2 + Na2CO3 === CaCO3��+ 2NaOH��
��3������ͼ����Ϣ��֪�������ɻ�������ʱ�������е���͵���pH�ı仯��ϵ�жϣ�������͵���pH���ﵽ9����ʱ���ɻ����������Ƶ�����Ϊ6�죬��ѡB��
��4��A���ɻ���������ɫ���γ������������йأ���ȷ��B���ɻ������кܸߵ�Ӫ����ֵ��Ӧ����ʹ�ã�����C�������ɻ������ܺ���������Ǧ����ͯ����ʳ�ã���ȷ��D������Ƥ���ɻ�����ҹ���ú���ܱ��ʣ�����ʳ�ã���������ѡAC��

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�����Ŀ����ѧ��ȤС����³��ʦ��ָ���½��п���̽��ʵ�飬���ǽ����ϵĻ��ͷ���ھƾ��Ƶ�о�ϣ���ȼ�ƾ��ƣ�����ķ��ֵ�о�ϵĻ��ͷ��Ȼû���ţ�����ʲôԭ���أ�����������һ��̽����
����������衿����1����о�����Ľ��紦�¶ȵ��ڻ��ͷ���Ż�㡣����2����о�����Ľ��紦����Ũ��̫�ͣ���֧�ֻ��ͷ��ȼ�ա�����3�����߾���Ӱ�����ء�
���������ϡ��ٻ��ͷ��Ҫ��KClO3��MnO2����ȼ�Pճ�ϼ�����ɣ��ú����߲����Dz�û��ͷ���Ż��Լ��303.4�档�ڻ��ͷ������Ũ��Ϊ12.2%�������в���ȼ�ա�

��ʵ����֤1��Ϊ����֤����1��С�����ø��´������ⶨ�ƾ��Ƶ�о�����Ľ��紦���¶ȣ���ͼ�����õ����������£�
�������� | ��1�� | ��2�� | ��3�� |
�¶�/�� | 236 | 243 | 240 |
��1��С�����ж�β�����Ŀ����___________��
��2�������ϱ���֪����о�ϻ��ͷδ���ŵ�ԭ����________________________��
��3����д�����ͷ��ȼ�����з�����Ӧ�Ļ�ѧ����ʽ�����������ṩ���ʣ�_______________�����뵽���ȼ��ʱ���ֵİ��̣�С���Ʋ���̵ijɷֿ�����______________��
��ʵ����֤2��Ϊ����֤����2��С����ע������ȡ�ƾ��Ƶ�о�����Ľ��紦�����壬��������������������������������������������
�������� | ��1�� | ��2�� | ��3�� | ƽ��ֵ |
������Ũ��/% | 5.93 | 5.29 | 6.53 | 5.92 |
��4������ʵ��1��2̽�����ݣ���֪����______________��ȷ��
��5��С�����ļ�������Ũ�ȴ��ڲ����ԭ�������__________________��
����˼���ۡ���ͼ��С��ͬѧ�����ͷ����װ�п������ܱյ�������ȼ�գ�����������������������˲���������������������Ũ�ȱ仯����ô��_______________���A��B��C��D������ʱ���ͷϨ��
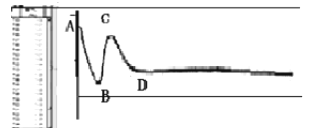
���������ѧ��������������A8�Ρ�BC�Ρ�CD������Ũ�ȱ仯��ԭ��_____________________����______________________����_______________________��