��Ŀ����
����Ŀ��������ͼ�ش����⣺

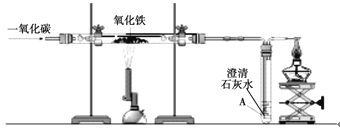
��1��װ��ͼ�б�����������ƣ��� ���� ��
��2��ʵ��������Aװ����ȡ��������Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽ�� ��
����G�ռ�������̼��������̼Ӧ�� ��ѡ��a����b��������װ��C��װ��B��ȣ�����Ҫ�ŵ��� ��
��4����ҵ����̼����ڸ����·ֽ�����ȡ�����ƺͶ�����̼��
����Ҫ��ȡ5.6t�����ƣ���̼��Ƶ������Ƕ��٣�����д��������̣�
��С��Ϊ�ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡһ������ʯ��ʯ��Ʒ����20gϡ�����4�μ�����Ʒ�У���Ʒ�г�̼����⣬����ɷֲ������ᷴӦ��Ҳ������ˮ������ַ�Ӧ�����ˡ�����Ȳ��������������������±���
ϡ��������� | ʣ���������� |
��һ�μ���5g | 1.5g |
�ڶ��μ���5g | 1.0g |
�������5g | 0.5g |
���Ĵμ���5g | 0.4g |
����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ ��
��Ҫ��ȡ5.6t�����ƣ���������Ҫ����ʯ��ʯ�������� ��
���𰸡���1���Թ� ����ƿ��2��2KClO3![]() 2KCl+3O2��
2KCl+3O2��
��3��CaCO3+2HCl==CaCl2+H2O+CO2�� a ���Կ��Ʒ�Ӧ�ķ�����ֹͣ
��4���ٽ�������̼��Ƶ�����Ϊx��
CaCO3![]() CaO+CO2��
CaO+CO2��
100 56
x 5.6t
100�U56=x�U5.6t x=10t
����̼��Ƶ�����Ϊ10t��
��80% ��12.5t
��������
�����������2��װ��A���̹̼����ͣ��Թܿ�û����һ�����������ü�������صķ�����ȡ������ͬʱ�������Ȼ��أ�2KClO3![]() 2KCl+3O2����
2KCl+3O2����
��3��ʵ������ʯ��ʯ��ϡ������ȡ������̼��ͬʱ�������Ȼ��ƺ�ˮ��CaCO3+2HCl==CaCl2+H2O+CO2����������̼���ܶȱȿ������Ⱦۼ��Ӽ���ƿ�ĵײ����ʶ�����̼�����a��ͨ�룻Cװ���пɽ�ʯ��ʯ���ڸ����ϣ�ϡ����ӳ���©�����룬������Ҫ��Ӧʱ����ֹˮ�м�ס��Ƥ�ܣ�ϡ������볤��©���У������Һ����룬��Ӧֹͣ������װ�õ��ŵ����ܿ��Ʒ�Ӧ�ķ�����ֹͣ��
��4�������û�ѧ����ʽ�����ݷ�Ӧ�������ȼ��ɼ����̼��Ƶ�������
��������̼��Ƶ�����Ϊx��
CaCO3![]() CaO+CO2��
CaO+CO2��
100 56
x 5.6t
100�U56=x�U5.6t
x =10t
����̼��Ƶ�����Ϊ10t��
�����ݱ�����Ϣ����5gϡ�����ܷ�Ӧ��0.5g̼��ƣ���֪̼�����Ʒ��������Ϊ2g�������0.4g����ʣ�࣬�����ʵ�����Ϊ0.4g����ô̼��Ƶ�����=2g-0.4g=1.6g��
̼��Ƶ���������=1.6g��2g ��100%=80%��
Ҫ��ȡ5.6t�����ƣ���������Ҫ����ʯ��ʯ������=10t��80%=12.5t