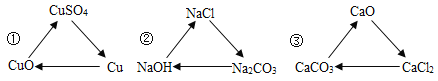
��Ŀ����
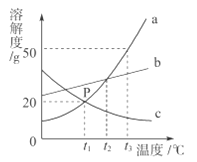
����Ŀ����10.0gijʯ��ʯ(���ʲ�����ˮ�Ҳ��μӷ�Ӧ)���Ⱥ�μ�100.0gϡHCl��һ������Na2CO3��Һ����Ӧ�����м�����Һ�������벻�ܹ������������������ϵ����ͼ��ʾ������˵����������
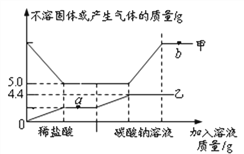
A. �ױ�ʾ���ܹ��������
B. a���Ӧ��Һ�е�����ֻ��1��
C. ��ϡ�����������������Ϊ7.3%
D. ����b���Ӧ��Һ�е����ʣ����ȵμ�������AgNO3��Һ�����ú��ٵμ�����Ca(NO3)2��Һ
���𰸡�BD
��������ʯ��ʯ�����ʲ�����ˮ�Ҳ��μӷ�Ӧ����ͼ��֪��10.0gijʯ��ʯ�м���������������Լ������ʣ��5g����Ϊ���ʣ���ʯ��ʯ��̼��Ƶ�����Ϊ5g��a�������������Ӧ��Һ�е����������ɵ��Ȼ��ƺ������Ȼ��⣬B����������Һ�м���̼����ʱ��̼�����������ᷴӦ�����Ȼ��ơ�������̼��ˮ��Ȼ�����Ȼ��Ʒ�Ӧ����̼��Ƴ������Ȼ��ƣ��ɹ�ϵʽ��CaCO3 ~ CaCl2~ Na2CO3~ CaCO3��֪���ɵ�̼�����ʯ��ʯ��ԭ��̼���������ͬ�����Լױ�ʾ���ܹ��������������Ϊ10g��A��ȷ����5g̼�����ȫ��Ӧ���ɶ�����̼������Ϊx,��Ӧ���Ȼ��������Ϊy
CaCO3 + 2HCl == CaCl2 + H2O + CO2��
100 73 44
5g y x
100/5g=44/x x=2.2g
100/5g=73/y y=3.65g
��ͼ���֪����̼���Ʒ�Ӧ������̼�������ﵽ4.4g��˵�������̼���Ʒ�Ӧ���ɶ�����̼2.2g������̼���Ʒ�Ӧ���Ȼ�������Ϊm
Na2CO3 + 2HCl == 2NaCl + H2O + CO2��
73 44
m 2.2g
73/m=44/2.2g m=3.65g
���������ʵ���������Ϊ�� ��3.65g+3.65g����100g��100%=7.3%��C��ȷ��
b���Ӧ��Һ�е�����Ϊ�Ȼ��ƺ�����̼����������ʱ��Ӧ�ȵμ�������HNO3��Һ������������֤����̼������ͬʱ����̼�������ų����Ȼ��Ƶ���֤�ĸ��������ú��ٵμ�����AgNO3��Һ�����ɰ�ɫ������֤�����Ȼ��ơ���D����ѡBD��

 ��ǰ����ϵ�д�
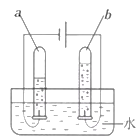
��ǰ����ϵ�д�����Ŀ����ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
[�������1] ������������Һ�Ƿ�������أ�
[ʵ��̽��1]
ʵ����� | ʵ������ | ʵ����� |
ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | _____________ | ����������Һһ�������ˡ� |
[�������2] ������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
[���������]
����1������������Һ���ֱ��ʡ� ����2������������Һȫ�����ʡ�
[��������] �� �Ȼ�����Һ�����ԡ�
�� �Ȼ�����Һ����̼������Һ��Ӧ(����ʽ)��________________________��
[ʵ��̽��2]
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ��________���� | ˵��ԭ��Һ��һ����̼���ơ� |
��ȡ������Թ��е������ϲ���Һ���μӷ�̪��Һ�� | _____________ | ˵��ԭ��Һ��һ����______�� |
[ʵ�����] ������������Һ_______(��������������ȫ����)���ʡ�
[��˼������] ������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ��_________��
��������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���________(����������������������)��
[������Ӧ��] ����������Һ���ױ��ʣ������ܷⱣ�档
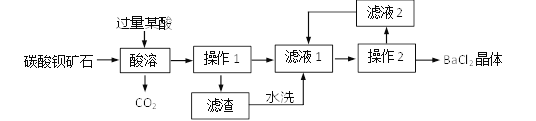
����Ŀ����ʡú̿��Դ�ḻ����˹������ú�㼰��Χ�Ҳ��У��Ǿ����к�������ܳƣ���Ҫ�ɷ��Ǽ��顣
(1)����д��������ȫȼ�յĻ�ѧ����ʽ��__________________________________��
(2)ú����˹��ը��������Ҫ������
����˹�����ڱ�ը���ķ�Χ�ڣ���________________________________________��
(3)�±��dz�����������ı�ը���ޣ�����ݴ��жϣ�����������ը��������____��
��ȼ���� | ��ը���ޣ���������� |
H2 | 4.0%~74.2% |
CH4 | 5%~15% |
CO | 12.5%~74.2% |
(4)����ͼ���У���ȼ�պͱ�ը�ص���____��

(5)ij�ִ����Ŀ����ˡ���Ũ����˹���缼��������Ч����˿������ܱߵ����������õ硣���ּȲ�ú�ַ��������������ŵ���___________________________________��