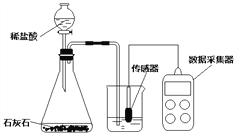
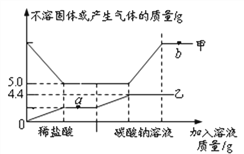
��Ŀ����
����Ŀ��ʵ������̼�ᱵ��ʯ(���ʲ�����ˮ�Ҳ��μӷ�Ӧ)�Ʊ�BaCl2�������Ҫ�������£�
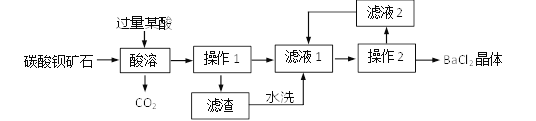
(1)ij��Ӧѡ��______(ѡ�����)��
a��H2SO4 b��HCl c��HNO3
(2)֤��ij������ķ��������ã����ϲ���Һ�еμ�______��Һ���������ݡ�
(3)����1�У����ò���������______�����������ձ���
(4)������ˮϴҺ����Һ2������Һ1�е�Ŀ����_____(ѡ�����)��
a�����ԭ�ϵ������� b�����ٱ��ζԻ�������Ⱦ
���𰸡� b ̼���� ©��(����ͨ©��������©��) a b
��������(1)��������Ҫ�Ʊ�BaCl2���壬��ѡ�����Ӧ���������������̼�ᱵ�����Ȼ��������ѡ�����������������������ᱵ�����ᱵ������ (2)֤����������ķ��������ã����ϲ���Һ�еμ�̼������Һ���������ݣ�(3)����1�ǽ������Թ�����Һ��������ǹ��ˣ����ò���������©�������������ձ���(4)������ˮϴҺ������Һ����Һ2�ж����Ȼ����������ؽ�������������Һ1�е�Ŀ�ļ������ԭ�ϵ������������ܼ��ٱ��ζԻ�������Ⱦ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij�ֿ�ʯ������þ��������������ͭ�Ͷ���������ɣ������Ʊ�������þ������ʾ��ͼ��ͼ��ʾ(��֪���������費����ˮҲ����ϡ���ᷴӦ)��
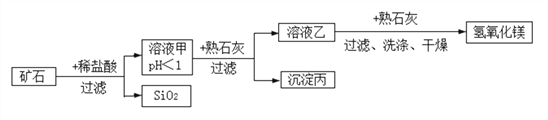
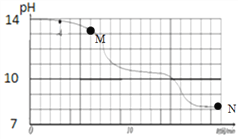
����Һ���м�����ʯ�ҵ�����Һ��pH������ʹ��Һ�еĽ�����������ת��Ϊ��������ʵ�������£�ʹ���������ӳ��������pH���ݼ��±���Ϊ��֤��Ʒ���ȡ����ٲ�Ʒ��ʧ�������ڲ�����������Һ�ҵ�pH��ȡֵ��һ���ķ�Χ��
�������� | Fe(OH)3 | Cu(OH)2 | Mg(OH)2 |
��ʼ������pH | 1.5 | 4.2 | 8.6 |
��ȫ������pH | 3.2 | 6.7 | 11.1 |
����˵���������
A. ��Һ���й���������������
B. ��Һ�ҵ�pH�ķ�Χ��6.7<pH<8.6
C. �������ijɷ���Fe(OH)3��Cu(OH)2��Mg(OH)2
D. ��Һ���м�����ʯ�ҷ����Ļ�ѧ��Ӧ����ʽ��MgCl2+Ca(OH)2= Mg(OH)2��+CaCl2
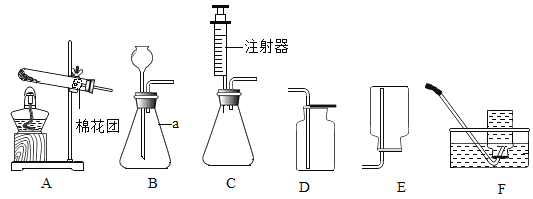
����Ŀ��ijʵ��С��ȡ����ʯ��ʱ������װ����ʯ�ҵ������Լ�ƿ�Ѿ��������ѣ�����С���еļ�λͬѧ�����еijɷֽ�����̽����

��������룩С����Ϊ���У�CaO��Ca(OH)2��
С����Ϊ���У�CaO��Ca(OH)2��CaCO3��
С����Ϊ���У�Ca(OH)2��CaCO3��
�����ʵ�飩
ʵ����� | ʵ������ | ���� | |
����һ | ȡ�������Թ��м�ˮ�� | Һ����ǣ��������Թ���ڣ��о������� | ______________________ |
����� | ����һ���Թ��еμ���ɫ��̪��Һ | ��Һ���ɫ | ______________________ |
������ | _______________________ | �Թ������������� | һ������CaCO3 |
���ó����ۣ�ͨ������̽����֪_________ͬѧ�IJ�����ȷ����д��ʹCaO���ʵĻ�ѧ����ʽ��______��
����չ�������ʯ����ȫ������Ϊ̼��ƣ��������ʵ�����֤��(д����Ҫ�IJ��衢����ͽ���)___��
����Ŀ���ճ������У���������̼������Һ���м��ԣ���ϴ�;��ϵ����ۣ�����Խǿ��ȥ���۵�Ч��Խ�á������Ƕ�Ӱ��̼������Һ���Ե�����չ��̽����
��̼���ƹ���Ͳ�ͬ�¶ȵ�ˮ�������������������ֱ�Ϊ2%��6%��10%��̼������Һ������������Һ��pH����¼�������±���
ʵ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
������������ | 2% | 2% | 2% | 6% | 6% | 6% | 10% | 10% | 10% |
ˮ���¶�/�� | 20 | 40 | 60 | 20 | 50 | 60 | 20 | 40 | 60 |
��ҺpH | 10.90 | 11.18 | 11.26 | 11.08 | 11.27 | 11.30 | 11.22 | 11.46 | 11.50 |
��������������ݻش�
(1)ȥ���۵�Ч����õ���___________(��ʵ����)��
(2)��һ���¶ȷ�Χ�����¶ȶ�̼������ҺpH��Ӱ���ǣ���̼������Һ������������ͬʱ��_________��
(3)Ҫ����̼������Һ��pH����Һ���������������ı仯��ϵ���ߣ���ѡ���һ��ʵ����___________(��ʵ����)��������___________��
(4)���ijʵ��С����õ����õ�ȥ��Ч��������֮ǰ��ʵ����ɣ����Գ��Ե�ʵ��������___________��
A��12%��60�� B��8%��40�� C��10%��70��
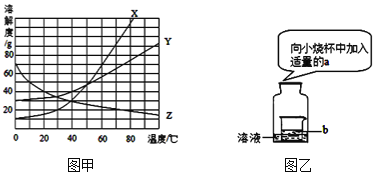
(5)��С������50g 10% Na2CO3��Һ������ͼ������ʾ:
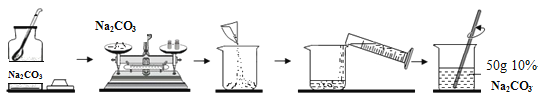
��С��Ӧ����Na2CO3������Ϊ__________g��������Na2CO3����ʱָ������ƫת����Ӧ____________________________ֱ����ƽƽ�⣮