��Ŀ����
����Ŀ��Ϊ�ⶨ�Ȼ��ƺ�̼���ƻ�������������ijС�����������ʵ�顣�ش��������⣺

��1��ʵ���з�����Ӧ�Ļ�ѧ����ʽΪ_____��
��2����������з�����Ӧ���ʵ�������x���ı���ʽΪ_____��
��3����������Ȼ��ƺ�̼�������������������Ϊ_____��
��4�������Ȼ�����Һ��������������Ϊ_____��
��5������30%���Ȼ�����Һ����ʵ�����õ��Ȼ�����Һ����Ҫ��ˮ_____��
��6����ҵ�����Ȼ���Ϊԭ�Ͽ����Ƶúܶ����ʣ��������������66.9t�����Եõ�_____t��̼���ơ�
���𰸡���1��![]()
��2��![]()
��3��117��106
��4��11.1%
��5��63g
��6��31.8g
��������
��1��ʵ����̼���ƺ��Ȼ��Ʒ�Ӧ����̼��Ƴ������Ȼ��ƣ���ѧ����ʽΪ��![]() ��
��
��2���⣺��̼��������Ϊx���Ȼ�������Ϊz����Ӧ�����Ȼ�������Ϊy��
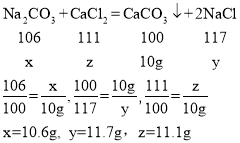
���![]() ��
��
��3�����ݼ���x=10.6g����ԭ��������Ȼ�������Ϊ��22.3g-10.6g=11.7g����˻�������Ȼ��ƺ�̼�������������������Ϊ��117��106��
��4����Ӧ����Һ���Ȼ�������Ϊ��11.7g+11.7g=23.4g��������������Ϊ10%������Һ����Ϊ��23.4g��10%=234g��
���������Ȼ�����Һ����Ϊ��234g+10g-22.3g-100g-21.7g=100g�����Ȼ�����Һ��������������Ϊ�� ![]() ��
��
��5���⣺����Ҫ30%�Ȼ�����Һ����Ϊm����Ϊϡ��ǰ�������������䣬��m��30%=11.1g��m=37g��������ˮ��100g-37g=63g��
��6����������66.9t�����ɵ�̼���Ƶ�����Ϊ��66.9t��![]() =31.8t��
=31.8t��

����Ŀ�����г�ȥ���ʵ�ʵ�������ȷ����(����)(������Ϊ����)
ѡ�� | ���� | ʵ����� |
A | CO2(CO) | �ڿ����е�ȼ |
B | CuO(Cu��) | �ڿ����м��� |
C | FeSO4��Һ(CuSO4) | ��������п�ۣ����� |
D | Zn(Fe) | ��������ϡ���ᣬ���� |
A. A B. B C. C D. D