��Ŀ����
����Ŀ�������������г�����һ�����ʣ�ij��ȤС��������ǵ������������̽����
���������룩С��ͬѧ��Ϊ��ɫֲ��ͨ��������ý�������̼��ˮת���������Ǻ������������ǽ�һ��ת��Ϊ���ǣ���������һ������̼����Ԫ�أ����ܺ�����Ԫ�ء�
��ʵ��̽����
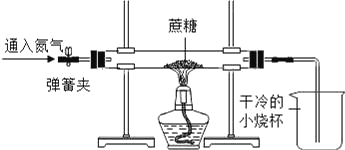
����һ��С�������Ƿ���������ȼ�գ���������������ˮ�Ͷ�����̼���ó���������������̼���⡢������Ԫ����ɵ�����С����Ϊ�÷�����������ʵ��ֻ��˵��������һ������_________Ԫ�ء�
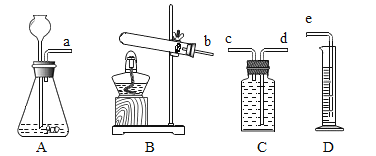
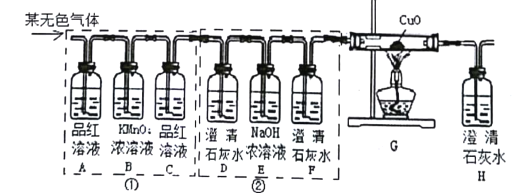
������������ͼ��ʾװ�ý���ʵ�飬ʵ�鿪ʼ��ͨ��һ���������Ȼ��رյ��ɼУ���ȼ�ƾ��Ƹ����Ǽ�ǿ�ȡ�
��1����ͨ��һ���������Ŀ����____________��
��2��ʵ���оƾ��Ƽ����ֵ�Ŀ����___________��
��3���۲쵽__________���֣�˵�������к�����Ԫ�غ���Ԫ�ء�
��4���۲쵽Ӳ�ʲ������в����к�ɫ���壬���²�ú�ɫ�����ǵ���̼��������������ͼ��ʾװ�ã���ѡ�Լ���������֤����̼��ʵ�顣ʵ����̺��������±���
ʵ����� | ʵ������ |
���ձ��е��������ij���ʯ��ˮ����Ӳֽ��������ͨ����������ȼ�ƾ��� | ��Ӳֽ�������еĺ�ɫ�������ȼ�գ������⣻ ��____________�� |
д������ʵ��������ձ��з�Ӧ�Ļ�ѧ����ʽ��________________��
��ʵ����ۣ���������̼���⡢������Ԫ����ɵġ�
��������֤��
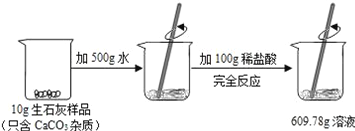
��֪����ѧ�仯�У����ӵ�������ı䣬��ԭ�ӵ����ࡢ�������������������ı䡣�ֳ�ȡ34.2 g���ǣ������������ȣ��������52.8g CO2��19.8g H2O����34.2 g�����и�Ԫ�ص���������̼Ϊ_______g������Ϊ_______g������Ϊ_______g���ݴ˿�֪���ǵĻ�ѧʽΪ________��ѡ����ĸ����
A. C6H5NO2 B. C6H12O6 C.Cl2H22O11
���𰸡�̼���� �ų�װ���еĿ��� ��£��������¶� �ձ��ڱ���ˮ�� ����ʯ��ˮ����� Ca��OH��2+CO2�TCaCO3��+H2O 14.4 2.2 17.6 C
��������
[ʵ��̽��]����һ��ʵ��ֻ��˵��������һ������̼����Ԫ�أ���һ��������Ԫ�أ�
����������1����ͨ��һ���������Ŀ�����ų�װ���еĿ������Է�ֹ�����еijɷ�Ӱ��ʵ������
��2��ʵ���оƾ��Ƽ����ֵ�Ŀ���Ǿ�£��������¶ȣ�
��3���۲쵽�ձ��ڱ���ˮ����֣�˵�������к�����Ԫ�غ���Ԫ�أ�
��4��С�ձ��м��������ij���ʯ��ˮ����Ӳֽ��������ͨ����������ȼ�ƾ��ƣ�Ӳֽ�������еĺ�ɫ�������ȼ�գ������⣬����ʯ��ˮ����ǣ�˵����Ӧ�����˶�����̼��ʵ�����������ʾ���ձ����������ƺͶ�����̼��Ӧ����̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
[������֤]��֪����ѧ�仯�У����ӵ�������ı䣬��ԭ�ӵ����ࡢ�������������������ı䡣�ֳ�ȡ34.2g���Ǹ����������ȣ��������528gCO2��19.8gH2O����34.2g�����и�Ԫ�ص���������̼Ԫ������Ϊ��52.8g��![]() =14.4g������Ԫ������Ϊ��19.8g��
=14.4g������Ԫ������Ϊ��19.8g��![]() =2.2g������Ԫ������Ϊ��34.2g-14.4g-2.2g=17.6g��������̼ԭ�ӡ���ԭ�ӡ���ԭ�Ӹ�����Ϊ��
=2.2g������Ԫ������Ϊ��34.2g-14.4g-2.2g=17.6g��������̼ԭ�ӡ���ԭ�ӡ���ԭ�Ӹ�����Ϊ��![]() ��
��![]() ��
��![]() =12��22��11���ݴ˿�֪���ǵĻ�ѧʽ��C12H22O11��
=12��22��11���ݴ˿�֪���ǵĻ�ѧʽ��C12H22O11��