��Ŀ����
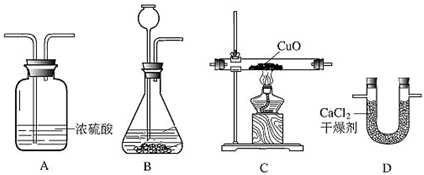
25�����ø����������������ԭ��������ͭ��ʵ��ⶨˮ��������ɣ���װ������ͼ��ʾ��

��ȫ��Ӧ����ʵ��ⶨ���������±����У�
�Իش�
��1��װ����ȷ������˳���������ң��ǣ�����ĸ��
��2��װ��B��������
��3������ʵ��������գ�ÿ�վ����г���ʽ���ó������������ˮ������Ϊ

��ȫ��Ӧ����ʵ��ⶨ���������±����У�
| ʵ��ǰ | ʵ��� | |
| ������ͭ+�����ܣ�������/g | 75.6 | 69.2 |
| ��������+U�ܣ�������/g | 110.8 | 118.0 |
��1��װ����ȷ������˳���������ң��ǣ�����ĸ��
AEBCD
����2��װ��B��������
��������
��װ��E����������ȥH2�е�HCl
����3������ʵ��������գ�ÿ�վ����г���ʽ���ó������������ˮ������Ϊ
7.2g
������ˮ����Ԫ�ص�����Ϊ6.4g
������ˮ����Ԫ�ص�����Ϊ0.8g
��ˮ���⡢����Ԫ�ص�������Ϊ1��8
���������������֪�������贿������ͨ��Eװ�ó�ȥ�Ȼ��⣬��ȡ�����������Ҫ������Ӧ�д�����ˮ������ʵ�����������ˮ��������������Ҫ�ѻ�ԭ����ͭ���ɵ�ˮ���ղ����أ����ӹ����л�Ҫ����ϴ����ͨ��˳��һ������ǣ�Һ��ϴ��װ�õ�ͨ��˳���dz����̳���Cװ�ü���������������ɵ�ˮ����Ԫ�ص�������U�ι����ؼ����ɵ�ˮ��������
����⣺��1������п����ϡ���ᷴӦ��ȡ����ʱ�õ�����������������ˮ�������Ȼ������壬������֪�����е��Ȼ������װ��E��ȥ����B C��������ˮ���������������װ���������ˮ�ֵ�װ�������������װ���ǿ��Ի����ģ�������˳��ΪAEBCD
��2��Bװ���Ƕ�����������ȥ�����е�ˮ������E�������dz�ȥ�����е��Ȼ������壮
��3��Cװ�ü������������CuO�е�Oʧȥ��������������������CuO�е�O������ˮ��U�ι����ص��������Ǹ÷�Ӧ���ɵ�ˮ����������ˮ��HԪ�ص���������ˮ��������ȥOԪ�ص�����������Ԫ������Ϊ��75.6 g-69.2 g=6.4g������ˮ������Ϊ��118.0 g-110.8 g=7.2g������ˮ����Ԫ�ص�����Ϊ 7.2 g-6.4 g=0.8g����ˮ��������������Ⱦ��ǣ�[��108.0g-100.8g��-��65.6g-59.2g��]����65.6g-59.2g��=1��8��
�ʴ�Ϊ��
��1��AEBCD
��2��������������ȥH2�е�HCl
��3��7.2g��6.4g��0.8g��1��8
��2��Bװ���Ƕ�����������ȥ�����е�ˮ������E�������dz�ȥ�����е��Ȼ������壮
��3��Cװ�ü������������CuO�е�Oʧȥ��������������������CuO�е�O������ˮ��U�ι����ص��������Ǹ÷�Ӧ���ɵ�ˮ����������ˮ��HԪ�ص���������ˮ��������ȥOԪ�ص�����������Ԫ������Ϊ��75.6 g-69.2 g=6.4g������ˮ������Ϊ��118.0 g-110.8 g=7.2g������ˮ����Ԫ�ص�����Ϊ 7.2 g-6.4 g=0.8g����ˮ��������������Ⱦ��ǣ�[��108.0g-100.8g��-��65.6g-59.2g��]����65.6g-59.2g��=1��8��
�ʴ�Ϊ��
��1��AEBCD
��2��������������ȥH2�е�HCl
��3��7.2g��6.4g��0.8g��1��8
����������Һ������ʹ�����Ҫ�����ܽ����̵��ܳ������������̳������ⶨˮ�������Ҫȷ�е�ʵ�����ݣ�����Ҫ�������ÿ��ʵ���Ŀ�ķ���ȷ�����⣬������ѧ���Ի�ѧ֪ʶ���ۺ�����������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
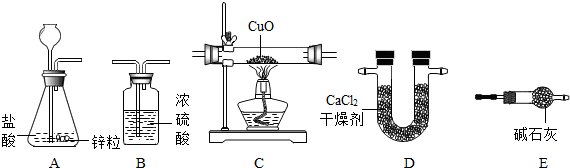
��6�֣����ø����������������ԭ����ͭ��ʵ��ⶨˮ��������ɣ�װ������ͼ��

װ�õ�����˳��ΪABCDE����ȫ��Ӧ����ʵ��ⶨ���������±����У�
|
|
ʵ��ǰ |
ʵ��� |
|
|
C������ͭ+�����ܣ�������/g |
75.6 |
69.2 |
OԪ�ص�����6.4g |
|
D���Ȼ���+U�ܣ�������/g |
110.8 |
118.0 |
H2O������ 7.2g |
�Իش�
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��װ��B�������� ��
��2��װ��C�е�����Ϊ ��
��3������ʵ��������գ�ˮ���⡢������Ԫ�ص�������Ϊ ��
��4��װ��E�м�ʯ��������ˮ������������װEװ�ã���ʹ�������Ԫ�ص����� �����ƫ��ƫС��������Ӱ�족֮һ���Լ����ж����� ��
���ø����������������ԭ����ͭ��ʵ��ⶨˮ��������ɣ�װ����ͼ��װ�õ�����˳��ΪABCDE����ȫ��Ӧ����ʵ��ⶨ���������±����У�

| ʵ��ǰ | ʵ��� | ||
| C������ͭ+�����ܣ�������/g | 75.6 | 69.2 | OԪ�ص�����6.4g |
| D���Ȼ���+U�ܣ�������/g | 110.8 | 118.0 | H2O��������7.2g |
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ________��װ��B��������________��
��2��װ��C�е�����Ϊ________��
��3������ʵ��������գ�ˮ���⡢������Ԫ�ص�������Ϊ________��
��4��װ��E�м�ʯ��������ˮ������������װEװ�ã���ʹ�������Ԫ�ص�����________�����ƫ��ƫС��������Ӱ�족֮һ���Լ����ж�����________��