��Ŀ����
ij��ȤС���ʳ�ô����ʳ��С�մ����ַ�ĩ��������̽����
[��������]
| ���� | ʳ�ô��� | ʳ��С�մ� |
| ��Ҫ�ɷ� | Na2CO3 | NaHCO3 |
| ����� | ˮ��Һ�Լ��� | ˮ��Һ�Լ��� |
| ���ȶ��� | ���Ȳ��ֽ� | 270��ʱ��ȫ�ֽ�Ϊ̼���ơ�������̼��ˮ |
[ʵ��̽��]
��1��̽������ˮ��Һ����ԵIJ���
С���ֱ����Ũ�ȵ�������Һ�е����̪��Һ���������߶�������ɫ����ʳ�ô�����Һ����ɫ����ɴ��Ʋ����������Һ���Ը�ǿ��С����ΪҪ�Ƚ�������Һ�ļ���ǿ������ֱ�����������вⶨ��
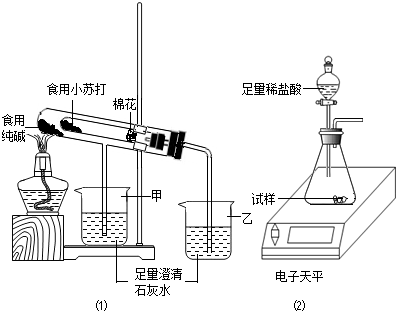
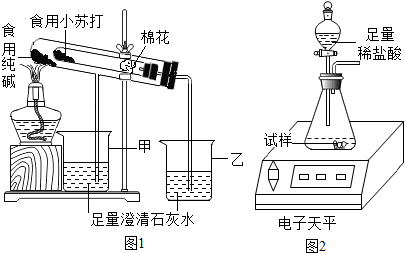
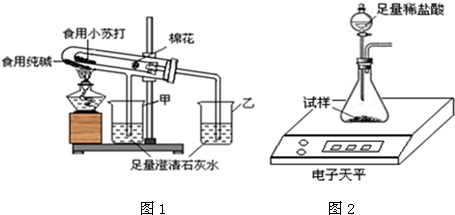
��2����֤���ַ�ĩ���ȶ���
����ʦ��ָ���£���С�鰴��ͼ1װ�ý���ʵ��������۲쵽��������ס����ҡ����ձ��г���ʯ��ˮ����ǣ��Թ��з�����Ӧ�Ļ�ѧ����ʽΪ������װ������������������
��3���ⶨʳ�ô����ĩ�Ĵ���
ȡʳ�ô����ĩ������������ͼ2װ�ý���ʵ��ⶨ��

���ݼ�¼���£�
| ������Ŀ | ����ʱ�� | ����/g |
| ���� | 11.0 | |
| װ��+ϡ���� | 160.0 | |
| װ��+ϡ����+���� | ��Ӧ��ʼ��20s | 167.0 |
| װ��+ϡ����+���� | ��Ӧ��ʼ��30s | 166.6 |
| װ��+ϡ����+���� | ��Ӧ��ʼ��90s | 166.6 |
�ٷ�Ӧ����CO2������Ϊ����g��
��ͨ������ȷ��ʳ�ô����ĩ��Na2CO3��������������д��������̣��������һλС������
�������õĽ����ʵ�ʴ��ȸߣ����ܵ�ԭ��������������һ�ּ��ɣ�
| [ʵ��̽��]���ݱ��й���̼���ƺ�̼�����Ƶ�����Լ����ȶ������ṩ����Ϣ�������1����2���� ��3�����ݷ�Ӧǰ�������ı仯��������ɶ�����̼�����������ݻ�ѧ�����ǵļ������ö�����̼�����������Ӧ��̼���Ƶ������������õ�ʳ�ô����ĩ��Na2CO3������������ ����������ڻӷ��Է�������������ƫ���ԭ�� | |
| ��� | �⣺��1���ӱ��п��Կ���ʳ�ô����ʳ��С�մ��ˮ��Һ���Լ��ԣ�����̪����ʹ��̪��죬�Ҽ���Խǿ��ɫԽ�죬�ⶨ��Һ�����ǿ���̶ȿɲ���pH��ֽ��PH����ɣ� ��2����ͼ���������ߵ����ȶ��Խ��з�����֪��ʳ��С�մ���Լ��ȷֽ����ɶ�����̼����ʳ�ô���ܣ����Է���װ�ÿ�֪���ձ��ij���ʯ��ˮ����ǣ���ֽⷽ��ʽΪ��2NaHCO3 ��3������ͼ�����ݷ�����֪11g��Ʒ��������ȫ��Ӧ�������仯�ǣ�11g+160g��﹣166.6g=4.4g����Ϊ�˹��̷����ķ�Ӧ��Na2CO3+2HCl=2NaCl+H2O+CO2�������Դ�����������ɶ�����̼�������� ��������4.4g������̼ʱ��Ҫ̼���Ƶ�������x Na2CO3+2HCl=2NaCl+H2O+CO2�� 106 44 x 4.4g
x=10.6g ����ʳ�ô����ĩ��Na2CO3����������Ϊ ��ͨ�����ϼ����֪�����õĽ����ʵ�ʴ��ȸߣ����Dz����Ķ�����̼������ʵ��ֵƫ���ܵ�ԭ����ϡ����ӷ�����HCl������CO2�����ų����Ӷ��������ⴿ��ƫ�� �ʴ�Ϊ����1���죻ʳ�ô����Na2CO3����pH��ֽ�� ��2���ң� 2NaHCO3 ��3����4.4����ʳ�ô����ĩ��Na2CO3����������Ϊ96.4%����ϡ����ӷ�����HCl������CO2�����ų����Ӷ��������ⴿ��ƫ����ˮ������CO2�����ų����Ӷ��������ⴿ��ƫ�� |
��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д� Na2CO3+CO2��+H2O��װ���е����ŵ������ǣ���ֹ����ʱС�մ�NaHCO3����ĩ���뵼�ܣ�
Na2CO3+CO2��+H2O��װ���е����ŵ������ǣ���ֹ����ʱС�մ�NaHCO3����ĩ���뵼�ܣ� =
=
 ��100%��96.4%��
��100%��96.4%��