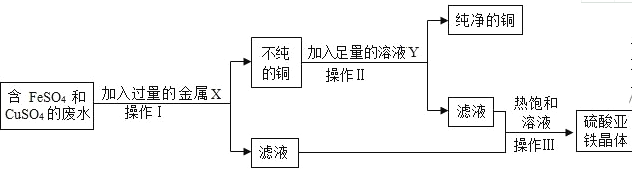
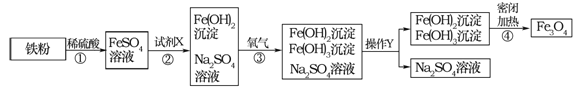
��Ŀ����
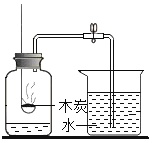
����Ŀ����ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�
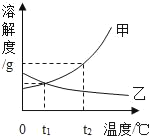
��1��_____��ʱ���ס����������ʵ��ܽ����ȣ�
��2���Ѽ����ʵIJ�������Һ��Ϊ������Һ�������й�˵����ȷ����_____��������ţ���ͬ��
A �ܼ�������һ����С
B ���ʵ��������ܲ���
C ���ʵ���������һ�����
D ��Һ������һ�����
E �ñ�����Һ�������ܽ���������
��3��ͨ������£���ʵ�����ù��������һ���������������ļ���Һ�����õ��������У�������ƽ��������У����ձ���_____��
A �ƾ��� B ҩ�� C ������ D ©�� E ����̨ F ��Ͳ G ��ͷ�ι�
���𰸡�t1 BE BCFG
��������
��1�������ܽ�����߿�֪��t1��ʱ���ס����������ʵ��ܽ����ȣ�
��2��A�������ʵIJ�������Һ��Ϊ������Һ���ܼ���������һ����С�������ý��µķ���ʹ�����ʵIJ�������Һ��Ϊ������Һ���ܼ����������ܲ��䣬��A����
B�������ʵIJ�������Һ��Ϊ������Һ�����ʵ��������ܲ��䣬�����ý��µķ���ʹ�����ʵIJ�������Һ��Ϊ������Һ�����ʵ��������ܲ��䣬��B��ȷ��
C�������ʵIJ�������Һ��Ϊ������Һ�����ʵ�����������һ����������ý��µķ���ʹ�����ʵIJ�������Һ��Ϊ������Һ�����ʵ������������ܲ�����С����C����
D�������ʵIJ�������Һ��Ϊ������Һ����Һ��������һ����������ý��µķ���ʹ�����ʵIJ�������Һ��Ϊ������Һ����Һ���������ܲ�����С����D����
E���ñ�����Һֻ�Dz����ܽ�����ʣ����п��ܻ������ܽ��������ʣ���E��ȷ��
��3��ͨ������£���ʵ�����ù��������һ���������������ļ���Һ�����õ��������У�������ƽ��������У����ձ���ҩ�ס�����������Ͳ����ͷ�ιܡ�

����Ŀ����ҵ����ͨ���������������ŷ�ǰ�辭����������ͼ�����ֳ����������к������ʵ�ת��·�������ֲ������ԣ���
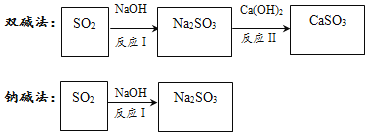
��1�����л���������SO2�йص���______������ĸ��ţ���
A ����B ����ЧӦC �����ն�
��2��˫��е���˫����ָ����______���ѧʽ����
��3��SO2��NaOH��Ӧ�Ļ�ѧ����ʽΪ______��
��4����ӦII�Ļ�ѧ��Ӧ����������______��Ӧ��ѡ�������������ֽ������û��������ֽ������÷�Ӧ�Ļ�ѧ����ʽΪ______��
��5����֪����ԭ�ϵļ۸������ʾ��
�Լ� | Ca��OH��2 | NaOH |
�۸�Ԫ/kg�� | 0.36 | 2.90 |
���ֹ����У�������ͬ����SO2��Ӧѡ��Ĵ���������______��ԭ����______��