��Ŀ����
����Ŀ����ѧ������������أ���ѧ�����������ߣ�
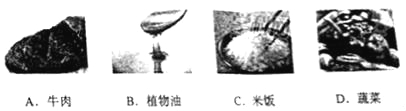
��1������ʳ���и���������� ������ţ���
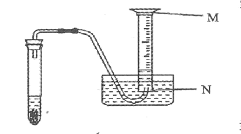
��2��ij��ȤС�����ˮ����ˮ��״�����е����о���ȡ��ˮ������ʵ���Ҿ��ú���ˣ�������Ҫ�õ��IJ����������ձ���©���� ��
��3����Ҫ�ⶨ��ˮ����ˮ�����ȣ������˵��� ������ţ�
A����ɫʯ����Һ B��pH��ֽ C����ɫ��̪��Һ
��4����ɭ�ֻ�ݳ���������ʱ���Ȼ�Ĵ�ʩ֮һ�Dz�������Χһ�����ȵ���ľ���Ӳݣ��γ�һ��������ǽ������ԭ���� ��
��5���ռ�¶���ڿ����в��������տ����е�ˮ���������⣬���ᷢ�����ʣ������û�ѧ����ʽ�����ռ���ʵ�ԭ�� ��
���𰸡���1��C����2������������3��B����4�����߿�ȼ���5��CO2+2NaOH=Na2CO3+H2O��
����������1��A��ţ���и��������ʣ���ѡ�����B��ֲ�����и�����֬����ѡ�����C�����и������ۣ������������࣬��ѡ����ȷ��D���߲��и���ά���أ���ѡ�����2��������Ҫ�õ��IJ���������©�����ձ��Ͳ���������3�����ָʾ��ֻ��ָʾ��Һ������Զ����ܲⶨ��Һ�����ȣ��ⶨ��Һ������Ҫ��pH��ֽ����4����������Χһ�����ȵ���ľ���Ӳݣ��γ�һ��������ǽ������ʹ��ȼ�ﱻ���ߣ��Ӷ���𣻣�5������������������еĶ�����̼��Ӧ����̼���ƺ�ˮ�����ɵ�������̼���ƣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O��

����Ŀ��ij�ܱ���������X��������������̼�������ʣ���һ�������³�ַ�Ӧ����Ӧǰ����������������ݼ�¼���£�
���� | X | O2 | CO2 | H2O |
��Ӧǰ������/g | 46 | 128 | 1 | 0 |
��Ӧ�������/g | 0 | ���� | 89 | 54 |
�������ж��У���ȷ���ǣ� ��
A. ����X��̼����Ԫ����� B. ����X��̼���⡢��Ԫ�����
C. ��ַ�Ӧ��ʣ����������Ϊ16�� D. ����CO2��H2O����������89��54