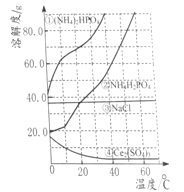
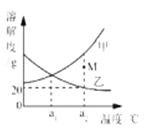
��Ŀ����
����Ŀ��ijͬѧ��ʵ������6.5g��п���������ʲ����뷴Ӧ����ϡ������ȡH2��
��1������100g 10%ϡ������Ҫ37%Ũ���ᣨ�ܶ�1.19g/cm3�������Ϊ mL���������С�����һλ�������˽�ͷ�ιܡ��ձ����Ҫ�IJ��������� �� ��
��2��6.5g��п��ȫ��Ӧ������H2������Ϊ0.16g����ô�п��п����������Ϊ ��
��3����֤����װ��ˮ��10mL��Ͳ����С�Թ��ռ�H2��װ����ͼ��ʾ��
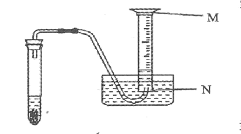
��10mL��Ͳ�����̶ȿ��� �ˡ�����д��M����N����
����10mL��Ͳ�е�ˮȫ�����ž���ʵ���ռ�����H2���V 10.0mL������д��������������������
���𰸡���1��22.7 ��Ͳ����������2��80%��3��N����
��������
��1��ϡ��ǰ�����ʵ�������ȣ�����Ҫ37%Ũ��������ΪΪx��
100g��10%=x��1.19g/cm3��37% ���x=22.7cm3, ����ϡ�����õ��IJ��������У��ձ�������������Ͳ����ͷ�ιܡ���2����ô�п��п������Ϊy
Zn+2HCl==ZnCl2+H2��
65 2
y 0.16g
![]() =
=![]() ���y=5.2g��
���y=5.2g��
�ô�п��п����������Ϊ��![]() ��100%=80%
��100%=80%
��3����Ͳ�Ŀ̶ȴӵײ��������ռ�������������������ſ�ˮ�������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ