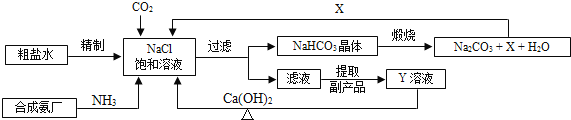
��Ŀ����
����Ŀ��������ͼ��ʾʵ��,�ش��������⣺
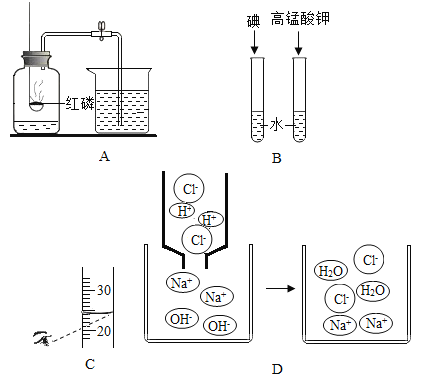
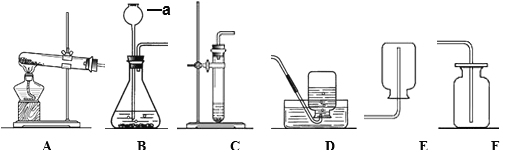
(1)��Aͼ��ʾʵ���У�ʵ���������뼯��ƿ��ˮ���������ƿ��ԭ������������֮һ����ɸ������ԭ����____________________________ (��һ��)��
(2)ͨ��Bͼʵ���е��������õ��Ľ�����____________________________��
(3)������һ�����������Ȼ�����Һʱ����ȡˮ�IJ�����Cͼ��ʾ��������������ȷ�� ��������Һ���Ȼ��Ƶ��������� ______ (����ƫ��������ƫС������������)��
(4)D ͼʵ���������ֵ���ʵ����____________________________��
���𰸡���ȼ����ǰ���ɼ�û�мн���Ƥ�ܻ��ȼ��������ƿ��ʱ̫�� �ܽ��������ʵ������й� ƫС �����������������ӽ�ϳ���ˮ����
��������
��1����ȼ����ǰ���ɼ�û�мн���Ƥ�ܣ�����ȼ��ʱ���ȣ��ᵼ�²�����������ͨ�������ݳ�����ȼ��������ƿ��ʱ̫�����ᵼ��ƿ�ڲ������������ݳ������ᵼ�½��뼯��ƿ��ˮ���������1/5�������ȼ����ǰ���ɼ�û�мн���Ƥ�ܻ��ȼ��������ƿ��ʱ̫����
��2������ˮ�м������ܽ⣻������ؿ�������ˮ����˵���ܽ��������ʵ������йأ�����ܽ��������ʵ������йأ�
��3����ȡˮʱ���Ӷ������ᵼ����ȡˮ�����ƫ�Ӷ�����������Һ����������ƫС�����ƫС��
��4�����۷�Ӧʾ��ͼ��֪���÷�Ӧ��ʵ���������������������ӽ�ϳ���ˮ���ӣ���Ӧǰ�������Ӻ������ӵ��������Ŀ�����䣻��������������������ӽ�ϳ���ˮ���ӡ�