��Ŀ����
����Ŀ��ij����ҩ�����Ч�ɷ�Ϊ������þ�����ǩ��ͼ��ʾ��Ϊ�ⶨ������þ�ĺ�����СϼͬѧȡһƬ��ҩ��(���Ϊ1 g)���ձ��У�����20 g������������Ϊ3.65%��ϡ���ᣬǡ����ȫ��Ӧ(����ҩ���������ɷֿ�����ˮ,���������ᷴӦ)��

(1)ͨ����֪�����ܼ����������___ (����ĸ���)��
A ÿƬҩ����������þ����������
B��Ӧ�������Ȼ�þ������
C��Ӧ����Һ������
D20g 3.65%��ϡ������ˮ������
(2)����ÿ����õĸ�ҩƬ��������þ������___��
���𰸡�ABCD 3.48g
��������
�������и�����ϡ�������������������������μӷ�Ӧ���Ȼ�����������ٽ�Ϸ���ʽMg(OH)2+2HCl=MgCl2+2H2O��ⷴӦ��������þ�����������ɵ��Ȼ�þ�������������������غ���ⷴӦ����Һ��������
��1��
A����ÿƬҩ����������þ������Ϊx����Ӧ���ɵ��Ȼ�þ������Ϊy��
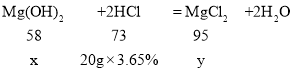
![]()
![]()
���x=0.58g
���y=0.95g
��������þ����������=![]() �ʷ������⣻
�ʷ������⣻
B�����Ϸ�����֪���ɵ��Ȼ�þ����Ϊ0.95g���ʷ������⣻
C��������þ��ϡ���ᷴӦ�����Ȼ�þ��ˮ��û������û�г������ɣ���Ӧ����Һ������Ϊ20g+1g=21g���ʷ������⣻
D������20g������������Ϊ3.65%��ϡ���ᣬ��֪�����Ȼ��������20g��3.65%=0.73g����ˮ������Ϊ20g-0.73g=19.27g���ʷ������⣻
����ABCD��
��2�����Ϸ�����֪ÿƬҩ�ﺬ������þ������Ϊ0.58g������˵��ÿ�����6Ƭ����ÿ����õ�������þ��������6��0.58g=3.48g������3.48g������������Ϊ����ÿƬҩ���к�������þ����Ϊx��
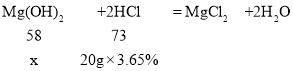
���x=0.58g����ÿ����õ�������þ����Ϊ6��0.58g=3.48g��
��ÿ����õĸ�ҩƷƬ������þ��������3.48g��

 ͬ������ϵ�д�
ͬ������ϵ�д�