��Ŀ����
����Ŀ��ʵ���ҳ�������װ������ȡ���ռ����塣��ش��������⡣

��1�������������ǣ�a__________________��b___________________,c__________________��
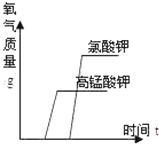
��2��ʵ������KMnO4��ȡ02��Ӧѡ�õķ���װ�ú��ռ�װ�õ����Ϊ____________������ĸ��ţ����÷���װ�õIJ���֮����____________________���÷�Ӧ�Ļ�ѧ����ʽ��________________��
��3��ijͬѧȡһ��Ũ��������ʯ��ʯ�Ʊ�������̼����Ӧ����ʽΪ________________________��
�����ɵ�����ͨ�����ʯ��ˮ�У�ʼ��δ�����ǣ����ܵ�ԭ����______________________��
����Ƽ�ʵ�������֤��
ʵ�鲽�� | ʵ������ | ʵ����� |
__________________________ | ________________________ | ����Ŀ���ԭ����� |
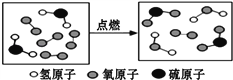
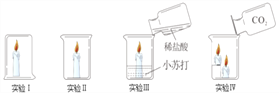
��4�������¿�״��ʯ��CaC2����ˮ��Ӧ������Ȳ ��C2H2��������������ƣ��÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________��ʵ������ȡ��Ȳʱ�����ϸ���Ƽ�ˮ�ٶȣ�������ҷ�Ӧ��������װ��ը�ѣ�ͼ���ʺ���ȡ��Ȳ����ķ���װ����_________����װ����ţ���
���𰸡� ��ƿ ��Һ©�� ˮ�� AF(��AH) �Թܿ�û��һ���� 2KMnO4 �� K2MnO4+MnO2+O2�� CaCO3+2HCl=CaCl2+H2O+CO2�� �������Ʊ��ʣ�������ӷ����Ȼ��⣩ ȡʯ��ˮ�ڽྻ�Թ��У������д���/�μӷ�̪ �������/����ɫ�����ɰ�ɫ������ CaC2+2H2O=C2H2+Ca(OH)2 CD
����������1�����ݳ������������2�����ݸ�������ڼ��ȵ������·ֽ���������ء��������̺����������3������̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��𣻸�������������Һ���ʣ�������ӷ����Ȼ��⣩��𣻸��ݶ�����̼��ʹ����ʯ��ˮ����ǣ��������ʯ��ˮ�еμӷ�̪��Һ�������4�����ݵ�ʯ��ˮ��Ӧ������Ȳ������������ƽ�𣻸��ݷ�Һ©����ע�����ܿ��Ƽ�ˮ�ٶȽ����1�������������ǣ�a��ƿ��b��Һ©��,cˮ�ۣ���2��ʵ������KMnO4��ȡ02����Ӧ���Ǹ�����ع��壬��Ӧ�����Ǽ��ȣ����ɵ������ܶȱȿ�����������ˮ����Ӧѡ�õķ���װ�ú��ռ�װ�õ����ΪAF(��AH)���÷���װ�õIJ���֮�����Թܿ�û��һ��������������ڼ��ȵ������·ֽ���������ء��������̺���������Ӧ�Ļ�ѧ����ʽΪ2KMnO4![]() K2MnO4+MnO2+O2������3��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2���������ɵ�����ͨ�����ʯ��ˮ�У�ʼ��δ�����ǣ����ܵ�ԭ�����������Ʊ��ʣ�������ӷ����Ȼ��⣩��ʵ�鲽�裺ȡʯ��ˮ�ڽྻ�Թ��У������д������μӷ�̪���������ɵ�����ͨ����������Һ����ʵ������ ��������ǣ�����ɫ���������ɰ�ɫ����������4����ʯ��ˮ��Ӧ������Ȳ������������ƣ���Ӧ�Ļ�ѧ����ʽΪCaC2+2H2O=C2H2+Ca(OH)2��ʵ������ȡ��Ȳʱ�����ϸ���Ƽ�ˮ�ٶȣ�������ҷ�Ӧ��������װ��ը�ѣ�ͼ���ʺ���ȡ��Ȳ����ķ���װ����CD��
K2MnO4+MnO2+O2������3��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2���������ɵ�����ͨ�����ʯ��ˮ�У�ʼ��δ�����ǣ����ܵ�ԭ�����������Ʊ��ʣ�������ӷ����Ȼ��⣩��ʵ�鲽�裺ȡʯ��ˮ�ڽྻ�Թ��У������д������μӷ�̪���������ɵ�����ͨ����������Һ����ʵ������ ��������ǣ�����ɫ���������ɰ�ɫ����������4����ʯ��ˮ��Ӧ������Ȳ������������ƣ���Ӧ�Ļ�ѧ����ʽΪCaC2+2H2O=C2H2+Ca(OH)2��ʵ������ȡ��Ȳʱ�����ϸ���Ƽ�ˮ�ٶȣ�������ҷ�Ӧ��������װ��ը�ѣ�ͼ���ʺ���ȡ��Ȳ����ķ���װ����CD��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������ճ��ر���Ʒ֮һ������Ҳ�ǻ�����Ⱦ��һ����Ҫ��Դ��������ij��ȤС�����÷Ͼ�п�̸ɵ����Ϊԭ�ϣ����������̽���Ĺ��̡�
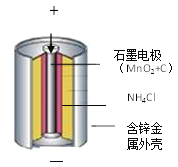
��֪ʶ������(1)п�̵�صĹ�������(��ͼ)��
(2)�ᾧˮ������������ʣ����������¶������£��ᾧˮ������ʧȥ���ֻ���ȫ���ᾧˮ������ɫ�ĵ�������(CuSO4 5H2O)����ʱ��ʧȥ�ᾧˮ��Ϊ��ɫ����ˮ����ͭ��ĩ(CuSO4)��
I.�Ʊ�𩷯����(ZnSO4xH2O)
С��ͬѧ�ι���ij���շϾ�п�̵�صĹ���������չ���������ͼ��
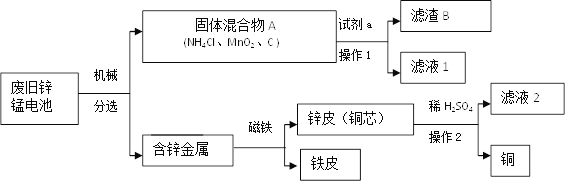
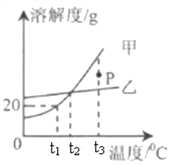
������ͼ���Լ�a�Ļ�ѧʽ��__________���õ�����Һ1ũҵ�Ͽ�����_____________��
(2)������B�ڿ����г������ ���ᴿ�ƵõĹ�����___________���÷����ᴿ��ԭ����(�û�ѧ����ʽ�ش�)____________________________��
�ǽ���Һ2��������Ҫ������п�����й��ܽ�Ⱥ��¶ȹ�ϵ���±���
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
����Һ2����Ũ����__________���ɵõ�𩷯����(ZnSO4xH2O)��
II.𩷯�����нᾧˮ�����IJⶨ
С��ͬѧ������𩷯����(ZnSO4xH2O)����ʵ���ң�����ͼװ�òⶨ�����нᾧˮ�ĺ���(ͼ�а�Ĥ��������ͨ���ֿɷ�ֹ�����ĩ���뵼��)��
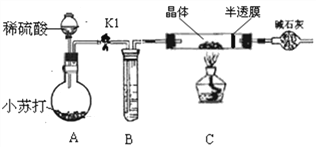
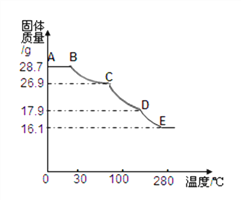
�ⶨ��������ȡ28.7g��������Cװ�õ�Ӳ�ʲ������У���������ȫʧȥ�ᾧˮ:(ZnSO4xH2O == ZnSO4 + xH2O)����ȴ�����º����������й���������Ϊ16.1g��
��A�еĻ�ѧ����ʽ��_____________��B�е��Լ��ɴ�����������ѡȡ�����ѡ����__________________��
A.Ũ���� B.��������Һ C.����̼������Һ D.����ʯ��ˮ
��ʵ�����������ͨ��CO2��õĽ����________(����ƫ������ ��ƫС��������Ӱ����)��
����ʵ����������𩷯�����нᾧˮ��xֵ��������̣�__________________
�ʽ�����𩷯������Ȼ���ʧȥ���ֽᾧˮ�����ȹ������йز���������������ͼ��д��D-E�η�����Ӧ�Ļ�ѧ����ʽ________________