��Ŀ����
����Ŀ��ʳƷ�뽡������Դ�뻷�������ǹ�ͬ��ע��������⡣
��1������Ӫ���ḻ����������ı������ܡ������и������ۡ�ά����C��ά����B�������ơ����ȡ�
������ġ����������ơ���ָ_____________������ĸ����
A��ԭ�� B������ C��Ԫ�� D������
������ȱ���������ᵼ��_____________��֢��
���������ܸ������ṩ������������_____________��
����Ԫ�ر���Ϊ����������������Ԫ�صIJ�����Ϣ��ͼ������˵����ȷ����____������ĸ����
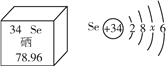
A�������ڽ���Ԫ��
B��һ����ԭ������34������
C������ԭ�ӽṹʾ��ͼ��x =18
D����Ԫ�ص����ԭ������Ϊ78.96 g
��2����Ȼ��������ʹ�õ�ȼ��֮һ������Ҫ�ɷ�ȼ�շ�Ӧ�Ļ�ѧ����ʽ��_____________��
��3��������̼���������ЧӦ����Ҫ���壬�ӡ���̼���ĽǶȷ�����Ӧ�������ٶ�����̼���ŷš����ŷų��Ķ�����̼���ղ�ת��Ϊ�������õ������ǿ�ѧ���о��ķ��������պ���������Ƿ��ж�����̼�����ݷ�Ӧ�Ļ�ѧ����ʽ��_____________��
���𰸡� C ƶѪ ���� BC CH4+2O2![]() CO2+2H2O Ca��OH��2+CO2�TCaCO3��+H2O
CO2+2H2O Ca��OH��2+CO2�TCaCO3��+H2O
����������1�������������������������ָԪ����������ȱ���������ᵼ��ƶѪ��֢�����������ܸ������ṩ������������������ۣ���A���������ڽ���Ԫ�أ�������B��һ����ԭ������34�����ӣ���ȷ��C������ԭ�ӽṹʾ��ͼ��x=18����ȷ��D����Ԫ�ص����ԭ������Ϊ78.96��������BC����2������ȼ�յĻ�ѧ����ʽΪ��CH4+2O2![]() CO2+2H2O����3��������̼��ʹ�����ʯ��ˮ����ǣ�����Ϊ������̼��ʯ��ˮ�е��������Ʒ�Ӧ������̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
CO2+2H2O����3��������̼��ʹ�����ʯ��ˮ����ǣ�����Ϊ������̼��ʯ��ˮ�е��������Ʒ�Ӧ������̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��

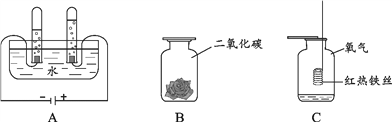
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ˮ��������ԴȪ��Ҳ�Dz���ȱ�ٵ���Դ��
(1)ij��Ȫˮ����Ҫ�����ʳɷּ����������
�ɷ� | Ca | K | Zn | F |
����(mg/L) | 20 | 3 | 0.06 | 0.02 |
����Ca��K��Zn��F��ָ_____��(�������ʡ�Ԫ�ء����ӻ�ԭ����)��
(2)ˮ��Ⱦ�������أ�ˮ��Դ�ı����ͺ����������ܵ����ǵ��ձ��ע����������������й����⣺
(��)����ij����ˮ��Ӳˮ������ˮ�����õ�������_____��ʵ���ҳ���_____�ķ���������ˮ��Ӳ��
(��)ijѧУ��ˮ�����Խ�����ˮ����Ϊ����ˮ�����д���������ͼ��ʾ��
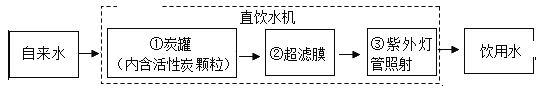
�ٶ�Ӧ��������_____(����ĸ���)�۶�Ӧ��������_____��
A��ɱ������������������B���������ʡ�����������C���������ˡ���������D������
(��)�������������ˮ����Ⱦ����_____��
A����ҵ��ˮֱ���ŷš���������������������B����ҵ�����������ŷ�
C����ֹʹ�ú���ϴ�·ۡ�����������������D������ʹ�û��ʡ�ũҩ