��Ŀ����
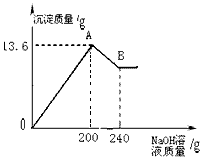
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al��OH��3��Ȼ��Al��OH��3���ܼ����������NaOH�������·�Ӧ��Al��OH��3+NaOH�TNaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش���1��A��ij�����Ļ�ѧʽ��______��B��ij�����Ļ�ѧʽ��______��
��2��ԭ�������MgCl2��AlCl3��������Ϊ���ٿˣ�
���𰸡���������1�������Ȼ�þ���Ȼ�������������������������þ�����������������з�����
����������������������Ʒ�Ӧ����ƫ�����ƽ��з�����
��2���������ɵij������ͼ�����������Ƶ��������з�����
����⣺��1���Ȼ�þ���Ȼ����м����������ƺ�����������þ���������������������������ƹ�������������������������������ƫ�����ƣ�����ֻʣ������þ�������ʴ�Ϊ��Mg��OH��2��Al��OH��3��Mg��OH��2
��2����������������������������ΪX�����Ȼ�����Ӧ��������������Ϊy�����Ȼ�þ��Ӧ���������Ƶ�����Ϊz��
Al��OH��3+NaOH=NaAlO2+2H2O
78 40
x 40g×10%
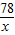 =
=
x=7.8g
AlCl3+3NaOH=Al��OH��3��+3NaCl
133.5 78
y 7.8g
 =
=
y=13.35g
MgCl2+2NaOH=Mg��OH��2��+2NaCl
95 58
z 13.6g-7.8g
 =
=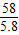
z=9.5g
��AlCl3Ϊ13.35g��MgCl2Ϊ9.5g��
�������ڽ������ʱ�����ȷ�������ͼ�����ת�۵��ʾ�ĺ��壬Ȼ������������������ݺͷ�Ӧ����ʽ�г���ϵʽ���з������
����������������������Ʒ�Ӧ����ƫ�����ƽ��з�����
��2���������ɵij������ͼ�����������Ƶ��������з�����
����⣺��1���Ȼ�þ���Ȼ����м����������ƺ�����������þ���������������������������ƹ�������������������������������ƫ�����ƣ�����ֻʣ������þ�������ʴ�Ϊ��Mg��OH��2��Al��OH��3��Mg��OH��2
��2����������������������������ΪX�����Ȼ�����Ӧ��������������Ϊy�����Ȼ�þ��Ӧ���������Ƶ�����Ϊz��
Al��OH��3+NaOH=NaAlO2+2H2O
78 40
x 40g×10%
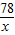 =
=
x=7.8g
AlCl3+3NaOH=Al��OH��3��+3NaCl
133.5 78
y 7.8g
 =
=
y=13.35g
MgCl2+2NaOH=Mg��OH��2��+2NaCl
95 58
z 13.6g-7.8g
 =
=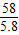
z=9.5g
��AlCl3Ϊ13.35g��MgCl2Ϊ9.5g��
�������ڽ������ʱ�����ȷ�������ͼ�����ת�۵��ʾ�ĺ��壬Ȼ������������������ݺͷ�Ӧ����ʽ�г���ϵʽ���з������

��ϰ��ϵ�д�
�����Ŀ
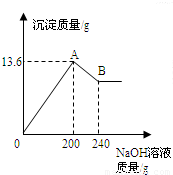
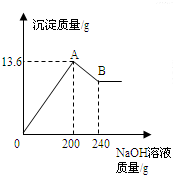 ��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�����������ͼ��ʾ���Իش𣺣���ʾ��Al��OH��3+NaOH�TNaAlO2+2H2O��NaAlO2������ˮ��
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�����������ͼ��ʾ���Իش𣺣���ʾ��Al��OH��3+NaOH�TNaAlO2+2H2O��NaAlO2������ˮ��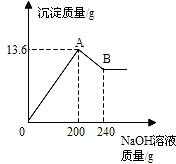 ��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al��OH��3��Ȼ��Al��OH��3���ܼ����������NaOH�������·�Ӧ��Al��OH��3+NaOH�TNaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش�
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al��OH��3��Ȼ��Al��OH��3���ܼ����������NaOH�������·�Ӧ��Al��OH��3+NaOH�TNaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش�