��Ŀ����
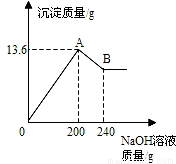
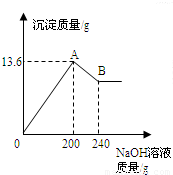
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al(OH)3��Ȼ��Al(OH)3���ܼ����������NaOH�������·�Ӧ��Al(OH)3+NaOH====NaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش�
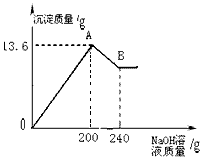
��1��A��ij�����Ļ�ѧʽ�� �� B��ij�����Ļ�ѧʽ�� ��
��2��ԭ�������MgCl2��AlCl3��������Ϊ���ٿˣ�
��2��ԭ�������MgCl2��AlCl3��������Ϊ���ٿˣ�
�⣺
��1��Mg(OH)2��Al(OH)3�� Mg(OH)2
��2��Al(OH)3 + NaOH = NaAlO2 + 2H2O
78 40
x 40g��10%
x = 7.8g
AlCl3 + 3NaOH = Al(OH)3��+ 3NaCl
133.5 78
y 7.8g
y = 13.35g
MgCl2 + 2NaOH = Mg(OH)2�� + 2NaCl
95 58
z 13.6g-7.8g
z = 9.5g
��AlCl3Ϊ13.35g��MgCl2Ϊ9.5g
��1��Mg(OH)2��Al(OH)3�� Mg(OH)2
��2��Al(OH)3 + NaOH = NaAlO2 + 2H2O
78 40
x 40g��10%
x = 7.8g
AlCl3 + 3NaOH = Al(OH)3��+ 3NaCl
133.5 78
y 7.8g
y = 13.35g
MgCl2 + 2NaOH = Mg(OH)2�� + 2NaCl
95 58
z 13.6g-7.8g
z = 9.5g
��AlCl3Ϊ13.35g��MgCl2Ϊ9.5g

��ϰ��ϵ�д�
 Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�
�����Ŀ
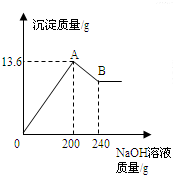 ��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�����������ͼ��ʾ���Իش𣺣���ʾ��Al��OH��3+NaOH�TNaAlO2+2H2O��NaAlO2������ˮ��
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�����������ͼ��ʾ���Իش𣺣���ʾ��Al��OH��3+NaOH�TNaAlO2+2H2O��NaAlO2������ˮ��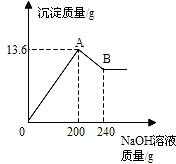 ��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al��OH��3��Ȼ��Al��OH��3���ܼ����������NaOH�������·�Ӧ��Al��OH��3+NaOH�TNaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش�
��MgCl2��AlCl3���ֹ�����ɵĻ��������������ˮ����������Һ����μ���10%��NaOH��Һ������NaOH��Һ�����������ɳ�������������ͼ��ʾ����֪��NaOH��AlCl3��Ӧʱ����ʹAlCl3ȫ��ת��Ϊ������ˮ��Al��OH��3��Ȼ��Al��OH��3���ܼ����������NaOH�������·�Ӧ��Al��OH��3+NaOH�TNaAlO2+2H2O������NaAlO2 �ǿ�����ˮ�����ʣ��Իش�