��Ŀ����
����Ŀ��Ŀǰ������ԴӶ���ȡ��Դ���ҹ�����Դ��ȡ����ȡ�ýϴ�ͻ�ơ�
��l������ȼ�ϵ����һ�����͵�ء������ɴӿ����л�ȡ��������ͨ�����·�Ӧ��ȡ�� 2NaCl+2H2O![]() 2X+Cl2��+H2�� ������X�Ļ�ѧʽ��_________��
2X+Cl2��+H2�� ������X�Ļ�ѧʽ��_________��
��2����ֹ2017��6��2�գ��ҹ����Ϻ���������������ɿ�ȼ������22�졣��ȼ������ѧʽCH4��8H2O�����ͷų��������壬��δ���ྻ������Դ����д������ȼ�յĻ�ѧ����ʽ��_______________��
��3��������ˮ����������֮���ת����ϵ����Ӧ���������ַ�Ӧ����ʡȥ�����������ڻ��Ϸ�Ӧ�Ļ�ѧ����ʽΪ______________�� CH4��H2O��Ca(OH)2��H2O
��4����ˮǹ����ԭ����________________��
���𰸡�NaOH CH4��2O2![]() CO2��2H2O CaO��H2O=Ca(OH)2 ���Ϳ�ȼ����¶ȣ���������
CO2��2H2O CaO��H2O=Ca(OH)2 ���Ϳ�ȼ����¶ȣ���������
��������
��1���������غ㶨�ɿ�֪����Ӧǰ�����Ԫ��ԭ�Ӹ������䣻������X�Ļ�ѧʽ��NaOH��
��2������ȼ�շ�Ӧԭ����CH4��2O2![]() CO2��2H2O��
CO2��2H2O��
��3��H2O��Ca(OH)2�У�ˮ�������Ʒ�Ӧ�����������ƣ�CaO��H2O=Ca(OH)2���ϡ����һ�������ڻ��Ϸ�Ӧ��
��4��ˮǹ����ԭ�������Ϳ�ȼ����¶ȣ�����������

 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
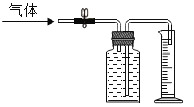
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�����Ŀ��ij��ѧ��ȤС����������װ�ý�����ȡ������ʵ�飬�����ѧ�����ݻش��������⡣
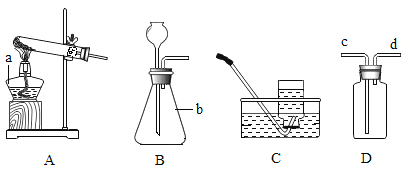
��1����ͼ��a��b���������ƣ�a_____��b_____��
���ø�����ع������������÷�Ӧ�ķ��ű���ʽ��_____������_____���������Ӧ���ͣ���ѡ�õ���ȡװ����_____��������ĸ��������Dװ�ý����ռ����������������IJ�����_____��
��2�����ȸ�����ع��塣�Ⱥ������ַ������ռ���ƿ�������ֱ�ⶨ���ռ���������Ũ�ȡ����ݼ�����
�ռ����� | ��ˮ�� | �����ſ����� | ||||
����Ũ��% | 80.3 | 90.0 | 89.8 | 79.9 | 79.6 | 79.7 |
����ƽ��Ũ��% | 86.7 | 79.7 | ||||
������ˮ���ռ�����ʱ���Թ۲쵽_____Ϊ���������ı���
������ˮ���ռ�ʱ��һ����������ƫ�ͣ� ���ܵȵ�_____��ʼ�ռ���ɱ�������������������ַ������ռ�������������������_____��
���������ſ������ռ���������Ũ��Ϊ80%���ҵ�ԭ����_____������ţ���
A �������ܶ��Դ��ڿ������ܶȣ��в��ֿ������Ÿɾ�
B ���۲쵽������ľ����ȼʱ������ƿ�ڻ��п���
C ���������뼯��ƿʱ��ƿ�ڿ����������������ɢ
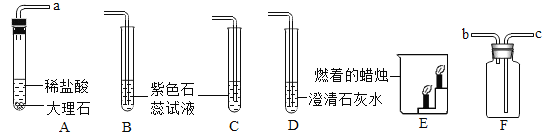
����Ŀ���������ʷе㲻ͬ����ʵ�ֻ����ķ��룬�����±������жϡ�
���� |
|
|
|
|
�е�/�� | -252.8 | -195.8 | -183.0 | -33.35 |
��1����ҵ����ȡ�����������¶���_____��<T<_____��ʱ�����Խ�Һ̬�����еĵ������������뿪��
��2��Ҫ����ҵ�ϳɰ��IJ��ﰱ����![]() �������ķ�Ӧ�ﵪ���������Ļ�����з��뿪���������˵��¶�Ӧ�ÿ�����_____��<T<_____����
�������ķ�Ӧ�ﵪ���������Ļ�����з��뿪���������˵��¶�Ӧ�ÿ�����_____��<T<_____����
��3����ҵ���Ʊ��������÷���Һ̬�����ķ���������_____������������������ѧ�����仯��
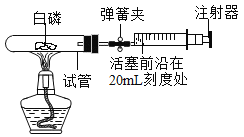
����Ŀ��ij��ȤС��ͬѧ��ʵ������ȡ������������������̽����
��1��Ϊ̽�����������������طֽ��ٶȵ�Ӱ�죬��������¶Ա�ʵ�飺
��3.0gKClO3��1.0gMnO2���Ȼ�ϼ���
�ڽ�Xg KClO3��1.0g����ͭ���Ȼ�ϼ��ȣ�����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
���з�Ӧ�ķ��ű���ʽ��___________������X��ֵΪ________��
��2����̽����Ӱ��˫��ˮ�ֽ��ٶȵ�ij�����أ�ʵ�����ݼ�¼�����
˫��ˮ������ | ˫��ˮ��Ũ�� | �������̵����� | ��ͬʱ���ڲ������������ | |
�� | 50.0g | 1% | 0.1g | 9mL |
�� | 50.0g | 2% | 0.1g | 16mL |
�� | 50.0g | 4% | 0.1g | 31mL |
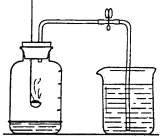
��ʵ���У�����O2��װ����_________������ţ���
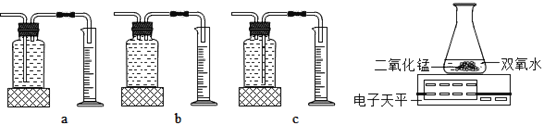
ʵ����ۣ�����ͬ������_________________��˫��ˮ�ֽ�ÿ졣����װ�õ�����ƽ����ʵ�飬ͨ���Ƚ�_____________________Ҳ�ܴﵽʵ��Ŀ�ġ�