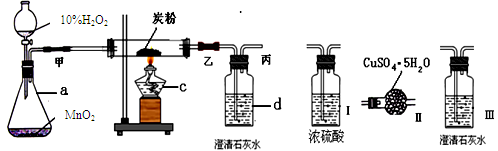
��Ŀ����
ѧϰ��ѧ��ͬѧ����ʶ�����������峺����ˮ������Ϊ����ˮ������Ӳ�Ƚϴ����Ի����ƫǿ��ˮ��������Ϊ����ˮ����ش��������⣺
��1��������ˮ�����ȣ���ʹ��___________��
A����ɫʯ����Һ B����ɫ��̪��Һ C��pH��ֽ
��2��������ƿ��ɫҺ�壬�ֱ�ΪӲˮ������ˮ������_____________�����Լ��������������ֿ�����
��3������Ӳˮ��ˮ���ڱڻḽ��һ��ˮ����ˮ�����������������塣������ͨ�����ʯ��ˮ����Ӧ�Ļ�ѧ����ʽΪ__________���۲쵽��������_________________________________________��
��1��C ��2������ˮ
��3��CO2+Ca(OH)2==CaCO3��+2H20 ,ʯ��ˮ�����
���������������1�����ָʾ��ֻ���ж���Һ������ԣ��ⶨ��Һ��������PH��ֽ�ⶨ��
��2��Ӳˮ�Ǻ��н϶�Ŀ����Ը�þ�������ˮ����������о�������������ˮû�У�
��3��ˮ������Ҫ�ɷ���̼��ƣ����ᷴӦ�����ɶ�����̼���壬������̼��ʹ�����ʯ��ˮ����ǡ�
���㣺��Һ������ԡ�ˮ��������̼������

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�ˮ������֮Դ���������ǵ�����������ء�
��1����ˮ�г��õ������������������� �������п��� ������ˮ��Ӳˮ��
��2����ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ���� ��
| A��������������ȼ��ֻ����ˮ | B��ˮ������ | C��ˮ�ĵ�� | D��ˮ�ľ��� |
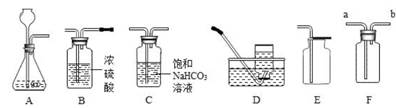

 2X+H2��+Cl2 ������X�Ļ�ѧʽ�� ���˽�ij�����ŷŷ�ˮ�����ȣ�����ʹ�� ���вⶨ��
2X+H2��+Cl2 ������X�Ļ�ѧʽ�� ���˽�ij�����ŷŷ�ˮ�����ȣ�����ʹ�� ���вⶨ��