��Ŀ����
����Ŀ��ˮ����������������������ء���ش��������⣺
��1��������ȷ��ˮ������Ԫ�غ���Ԫ����ɵ�ʵ����_____������ĸ����
A ������������ȼ������ˮ B ˮ������ C ˮ�ĵ�� D ˮ�ľ���
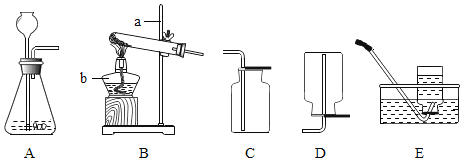
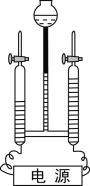
��2��������ͼ��ʾ�ļ���װ�ÿɰѺ�ˮת��Ϊ��ˮ��

����˵����ȷ����_____��
A С�����е�ˮΪ��ˮ
B ��õ�ˮ���ٶ����¶��й�
C �������е���Һһ�����Ȼ��Ƶı�����Һ
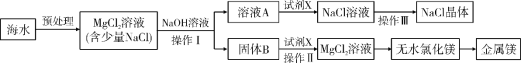
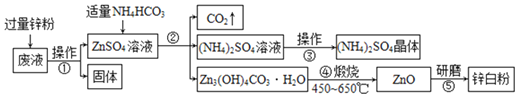
��3��ij�¹�������ˮ�Ʊ���������Ҫ�������£�
�����غϽ���_____��������������
�ڡ��豸1���еķ�Ӧ�ڳ����½��У��÷�Ӧ�Ļ�ѧ����ʽ��_____��
�ۡ��豸1���͡��豸2���л��ϼ۷����ı��Ԫ����_____��
��4��ijͬѧ����ͼ��ʾ��������ˮʱ����ˮ�м�������������ǿ�䵼���ԣ�������93.6g��������Ϊ5%����������Һ��ͨ��һ��ʱ��������ϲ���3.2g���������㣺
�ٵ�����ˮ������Ϊ���٣�_____(д���������)
�ڵ�����������Һ����������Ϊ_____��
���𰸡�AC AB ����� 2Al+3H2O=Al2O3+3H2�� Al��O��H 3.6g 5.2%
��������
��1��ˮ�����������������������������ʵ���Ԫ�ء�����������ʵ���Ԫ�ض�������ˮ����ˣ���˵��ˮ���⡢��Ԫ����ɣ���������ȼ�գ���ˮ�ĺϳɣ�Ҳ��˵��ˮ�����⡢��Ԫ����ɣ����AC��
��2��������ͼ��ʾ�ļ���װ�ÿɰѺ�ˮת��Ϊ��ˮ��
A��С�����е�ˮ��ͨ������õ���Ϊ��ˮ������ȷ��
B���¶�Ӱ��ˮ�����ٶȣ���õ�ˮ���ٶ����¶��йأ�����ȷ��
C���������е���Һ��һ�����Ȼ��Ƶı�����Һ���ʴ���
���AB��
��3�������غϽ����ɲ�ͬ������ɵģ�Ϊ����
�ڡ��豸1���еķ�Ӧ�ڳ����½��У���ͼ���Կ�����Ӧ��Ϊ����ˮ��������Ϊ����������������Ӧ�Ļ�ѧ����ʽ�ǣ�![]() ��
��
�ۡ��豸1���ķ�ӦΪ![]() ������Ԫ�غ���Ԫ�ػ��ϼ۸ı䣬���豸2���ķ�ӦΪ��
������Ԫ�غ���Ԫ�ػ��ϼ۸ı䣬���豸2���ķ�ӦΪ��![]() ������Ԫ�غ���Ԫ�ػ��ϼ۸ı䣬���ԡ��豸1���͡��豸2���л��ϼ۷����ı��Ԫ����
������Ԫ�غ���Ԫ�ػ��ϼ۸ı䣬���ԡ��豸1���͡��豸2���л��ϼ۷����ı��Ԫ���� ![]() ��
��![]() ��
��![]() ��
��
��4�����跴Ӧ��ˮ������Ϊy����
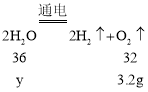
![]()
�𣺷�Ӧ��ˮ������Ϊ3.6g.��
�ڵ������������Ƶ�����Ϊ��93.6g��5%=4.68g.����Һ������Ϊ��93.6g-3.6g=90g��������Һ��������������Ϊ��![]() ��
��

 ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�