��Ŀ����
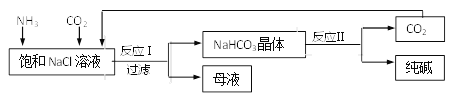
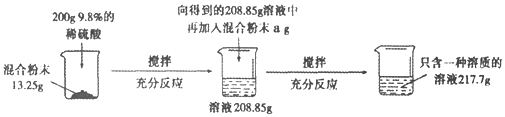
����Ŀ��ʵ������һ��̼���ƺ������ƵĻ�Ϸ�ĩ��Ϊ�ⶨ����̼���ƺ������Ƶ������ȣ�С��������ͼ��ʾ��ʵ�飺
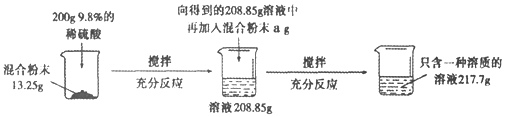
��ش��������⣺
(1)����ʵ������з�����Ӧ�Ļ�ѧ����ʽΪ____________________________��
(2)��13.25g��Ʒ�м���200gϡ������������������Ϊ_______________��
(3)������֪�������г������13.25g��Ʒ��Ӧ��ϡ��������������(x)�ı���ʽ________________��
(4)a����ֵ��_________��
(5)��Ϸ�ĩ��̼���ƺ������Ƶ�������Ϊ_________��
(6)��Ҫʹ����������Һ���ʵ�����������Ϊ10��������Ҫ�����м���ˮ������Ϊ____��
���𰸡� Na2CO3+H2SO4===Na2SO4+H2O+CO2�� 4.4g ![]() 13.25 4��1 119.3g
13.25 4��1 119.3g
��������(1)����ʵ������з�����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+H2SO4===Na2SO4+H2O+CO2����(2)����������غ㶨�ɣ���13.25g��Ʒ�м���20��ϡ������������������Ϊ13.25g+200g-208.85g=4.4g��(3) ����13.25g��Ʒ��Ӧ��ϡ��������������Ϊx��
Na2CO3+H2SO4===Na2SO4+H2O+CO2��
98 44
![]() 4.4g
4.4g
����ʽΪ�� ![]() ��
�� ![]() =9.8g�� (4)��Ӧ����Һ��ֻ��һ�����ʣ�˵��̼���ƺ�����ǡ����ȫ��Ӧ�����������������ʵ�����Ϊ200g
=9.8g�� (4)��Ӧ����Һ��ֻ��һ�����ʣ�˵��̼���ƺ�����ǡ����ȫ��Ӧ�����������������ʵ�����Ϊ200g![]() 9.8%=19.6g���ɴ˿�֪��Ӧ���ĵ��������ʵ�����Ϊ9.8g������13.25g��Ʒ��Ӧ��ϡ��������������Ϊ9.8g������a����ֵҲ��13.25��(5)��13.25g�������̼���Ƶ�����Ϊy��
9.8%=19.6g���ɴ˿�֪��Ӧ���ĵ��������ʵ�����Ϊ9.8g������13.25g��Ʒ��Ӧ��ϡ��������������Ϊ9.8g������a����ֵҲ��13.25��(5)��13.25g�������̼���Ƶ�����Ϊy��
Na2CO3+H2SO4===Na2SO4+H2O+CO2��
106 44
y 4.4g
![]() ��
�� ![]() =10.6g
=10.6g
���������Ƶ�����Ϊ13.25g-10.6g=2.65g����Ϸ�ĩ��̼���ƺ������Ƶ�������Ϊ��10.6g��2.65g=4:1��(6)���������ɵ������Ƶ�����Ϊz��
Na2CO3+H2SO4===Na2SO4+H2O+CO2 ��
98 142
19.6g z
![]() ��z=28.4g
��z=28.4g
�������μ����ĩ������Ϊ13.25g+13.25g=26.5g������26.5g��ĩ�������Ƶ�����Ϊ26.5g![]()
![]() =5.3g�����Է�Ӧ����Һ�����ʵ�����Ϊ28.4g+5.3g=33.7g����Ҫʹ����������Һ���ʵ�����������Ϊ10��������Ҫ�����м���ˮ������Ϊm����217.7g+m��
=5.3g�����Է�Ӧ����Һ�����ʵ�����Ϊ28.4g+5.3g=33.7g����Ҫʹ����������Һ���ʵ�����������Ϊ10��������Ҫ�����м���ˮ������Ϊm����217.7g+m��![]() 10%=33.7g��m=119.3g��
10%=33.7g��m=119.3g��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
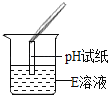
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ����ѧ��ȤС��ͬѧ����ʵ��̨�ϰ���ͼ��ʾ˳��ڷ���6ƿ��ͬ����ɫ��Һ(��ͼ��ʾ��A��B��C��D��E��F������Ӧ����Һ)������E��Һ���Լ�ƿ��ǩ����Ϊ�ˣ�������������̽����
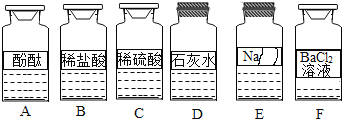
��������⡿E��Һ�ijɷ���ʲô��
�������жϡ����������ǩ��ʵ����ҩƷ����ڷ�ԭ��E��Һ��������_______(�����)����ڼ���������
��������롿
�������NaOH��Һ��
�������Na2CO3��Һ��
�������Na2SO4��Һ���������____��Һ(��дһ��)��
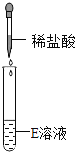
��Ʋ�ʵ����ȤС���ͬѧΪ��֤����������Ƿ���ȷ����Ʋ�������±���ʾʵ����
ʵ�鷽�� | ʵ��һ | ʵ��� | ʵ���� |
ʵ����� |
|
|
|
ʵ������ | ��ֽ��ɫ�����ձ�ɫ����pH��7 | ����ɫ��ζ������� | _____________�� �� |
ʵ����� | �������ȷ | ||
��������������
�������ʵ��һ�������ͬѧ����Ϊ�����������ȷ����ƽͬѧ��Ϊ�����������һ����ȷ������������_________________________��
����д����ʵ�������з�����Ӧ�Ļ�ѧ����ʽ___________________________��
����˼�����ۡ�
�������ۣ�ͬѧ����Ϊʵ���д������Բ������ô�����___________________��