��Ŀ����
�ҹ�����ר�Һ�°����������ġ������Ƽ���Ļ���ԭ���ǣ���Ũ��ˮ��ͨ�������Ķ�����̼����һ���Σ�Ȼ���ڴ���Һ�м���ϸС��ʳ�η�ĩ������̼�������ڸ�״̬���ܽ�Ⱥ�С���ʾ���������ͬʱ����̼�����Ʋ��ȶ������Ⱥ����ɴ��ˮ�Ͷ�����̼����������̼�����ƺ���Ȼ����Һ�м���ʳ�Σ�ʹ���е��Ȼ�淋����ᾧ�������Ȼ������ѭ��ʹ�ã���������������Ҫ�ش����⣺��1����������������ʵ��ʱ�����õ���ʼԭ��
��2�����ղ�Ʒ��
��3���йصĻ�ѧ����ʽ
��4��������Ϊ�����Ƽ���ŵ��������ĵ㣺
A�����������в��ֲ�Ʒ����Ϊ��ʼԭ��ʹ�ã�
B������Ʒ��һ�ֿ����õĵ��ʣ�
C����Ӧ����Ҫ���ȣ�
D������Ʒ������ɻ�����Ⱦ��
����Ϊ������ȷ���ǣ��ô��Żش�
������
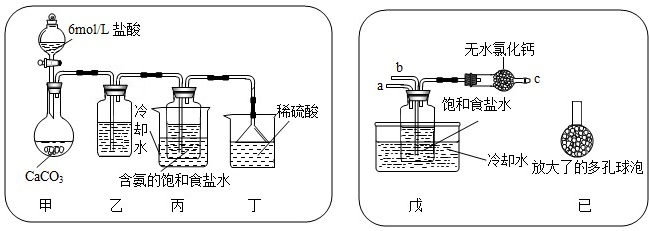
?����һ���μ�̼����泥�NH4HCO3��
NH4Cl+NaHCO3��NaHCO3?Na2CO3+CO2��+H2O��
������һ�����ķ�������ȷ������ԭ�Ͼ���Ũ��ˮ��������̼��NaCl�����ղ������NH4Cl��Na2CO3��������ѧ����ʽҲ�ɺ�����д������
|
| NaCl |
������һ�����ķ�������ȷ������ԭ�Ͼ���Ũ��ˮ��������̼��NaCl�����ղ������NH4Cl��Na2CO3��������ѧ����ʽҲ�ɺ�����д������
����⣺��1���ɡ������Ƽ���Ļ���ԭ����֪��������Ũ��ˮ�Ͷ�����̼��Ӧ����һ���Σ�NH4HCO3����
������Һ�м���ϸС��ʳ�η�ĩ������̼�����ƣ�̼�����Ʋ��ȶ������Ⱥ����ɴ��Na2CO3����
ˮ�Ͷ�����̼������ԭ�Ͽ���ѭ��ʹ�ã�ͨ��������֪����ʵ��ʱ�����õ���ʼԭ��Ϊ��NH3��CO2��NaCl��
��2����ʵ��ԭ����֪�������ķ�Ӧ�� NH3+H2O+CO2�TNH4HCO3��NH4HCO3+NaCl�TNH4Cl+NaHCO3
2NaHCO3
Na2CO3+CO2��+H2O �������ղ�Ʒ��Na2CO3��NH4Cl
��3���ɣ�2���ķ�����֪ʵ������з����ķ�Ӧ�У�
NH3+H2O+CO2�TNH4HCO3��NH4HCO3+NaCl�TNH4Cl+NaHCO3��2NaHCO3
Na2CO3+CO2��+H2O
��4���ɣ�2���ķ�����֪��Ӧ���ɵ�CO2����ѭ����Ϊ��ʼԭ��ʹ�ã���A��ȷ��
��Ϊ����Ʒ��NH4Cl�����е�Ԫ�أ�N�����ڵ��ʣ��� B��ȷ��
��Ϊ2NaHCO3
Na2CO3+CO2��+H2O �����Ӧ��Ҫ���ȣ���C����
��Ϊ����Ʒ��NH4Cl��CO2��H2O ������Ⱦ��������D��ȷ������ӦѡABD
�ʴ�Ϊ��
��1��NH3��CO2��NaCl��
��2��Na2CO3��NH4Cl
��3��NH3+H2O+CO2�TNH4HCO3 ��NH4HCO3+NaCl�TNH4Cl+NaHCO3 ��2NaHCO3
Na2CO3+CO2��+H2O
��4��ABD
������Һ�м���ϸС��ʳ�η�ĩ������̼�����ƣ�̼�����Ʋ��ȶ������Ⱥ����ɴ��Na2CO3����
ˮ�Ͷ�����̼������ԭ�Ͽ���ѭ��ʹ�ã�ͨ��������֪����ʵ��ʱ�����õ���ʼԭ��Ϊ��NH3��CO2��NaCl��
��2����ʵ��ԭ����֪�������ķ�Ӧ�� NH3+H2O+CO2�TNH4HCO3��NH4HCO3+NaCl�TNH4Cl+NaHCO3
2NaHCO3
| ||
��3���ɣ�2���ķ�����֪ʵ������з����ķ�Ӧ�У�
NH3+H2O+CO2�TNH4HCO3��NH4HCO3+NaCl�TNH4Cl+NaHCO3��2NaHCO3
| ||
��4���ɣ�2���ķ�����֪��Ӧ���ɵ�CO2����ѭ����Ϊ��ʼԭ��ʹ�ã���A��ȷ��
��Ϊ����Ʒ��NH4Cl�����е�Ԫ�أ�N�����ڵ��ʣ��� B��ȷ��
��Ϊ2NaHCO3
| ||
��Ϊ����Ʒ��NH4Cl��CO2��H2O ������Ⱦ��������D��ȷ������ӦѡABD
�ʴ�Ϊ��
��1��NH3��CO2��NaCl��
��2��Na2CO3��NH4Cl
��3��NH3+H2O+CO2�TNH4HCO3 ��NH4HCO3+NaCl�TNH4Cl+NaHCO3 ��2NaHCO3
| ||
��4��ABD
���������⿼������йغ����Ƽ���й�֪ʶ��ֻҪ���濴�⣬���������˺����Ƽ��ԭ��������ͻ�ӭ�ж��⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O