��Ŀ����
����Ŀ��ij���п���ѧʵ��������������У�
������60g 5%���Ȼ�����Һ��II������̼��ʵ������ȡ�����кͷ�Ӧ��������10mL ��ʳ��ˮ���ĸ���ǩ����ѧ����ǩȷ��һ��������п��졣
��1����ͬѧ��ǩ��ʵ���ң����ֱ���ʵ��������������������ҩƷ��
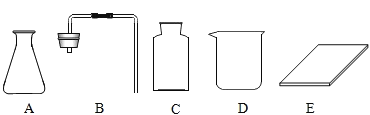
������������D������Ϊ________��
�ڼ�ͬѧ�鵽�Ŀ�ǩӦ����_______(����ĸ���)��
�������������巢��װ����ʵ���һ�������ȡ�������������壬����д����ȡ������Ļ�ѧ����ʽ__________��
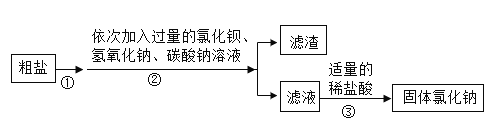
��2����ͬѧ�鵽�Ŀ�ǩ�Ǣ�����ʵ���������ͼ��ʾ����ش�
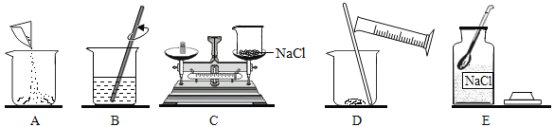
��ͼʾ�����У���һ���Ǵ���ģ���һ���������_______(��д��ĸ���)��
������������ȷ��˳����_______��(��д��ĸ���)��
�۰�����ȷ�IJ��������ƺõ���Һװ���Լ�ƿʱ�������Ƴ��˼��Σ����ʹ��Һ�����ʵ���������_______(����ƫ������ƫС������������)��
��3����ͬѧ�鵽�Ŀ�ǩ��IV�����ڽ���ʵ��ʱ�������õ�����Һ���ǣ����������ͬѧ����һ�²����������ԭ��_______��
���𰸡��ձ� II 2H2O2![]() H2O+O2�� C ECADB ���� ��ֽ���𣨻��������ɾ���©����Һ�泬����ֽ��Ե��
H2O+O2�� C ECADB ���� ��ֽ���𣨻��������ɾ���©����Һ�泬����ֽ��Ե��
��������
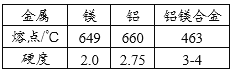
��1����ͨ������������ָ���������ÿ�֪��D���ձ���
��A����ƿ��B�ǵ����������ܣ� C�Ǽ���ƿ��D���ձ���E�Dz���Ƭ��һ������������������Һ����������������������ƽ��ҩ�ס���Ͳ����ͷ�ιܡ��ձ�������������������ֻ���ձ����ã�������̼��ʵ�����Ʒ�����ϡ���������ʯ����ʯ��ʯ������Ҫ�ɷֶ���̼��ƣ���Ӧ����ȡ������װ���ǹ����Һ���ڳ����µķ�Ӧ����Ϊ������̼������ˮ���ܶȱȿ��������Բ��������ſ������ռ�������������������ƿ�������������ܣ�����ƿ������Ƭ���кͷ�Ӧ������������ձ�������������ͷ�ιܵȣ�����10mL��ʳ��ˮ��������������̨����Ȧ���ձ�����������©�����ʼ�ͬѧ�鵽�Ŀ�ǩӦ���ǣ�������̼����ȡ����ѡ��
�۷���װ�����ڹ�Һ�����͵�װ�ã���˿�����ʵ�����ù���������Һ�Ͷ���������ȡ���������ж��������Ǵ�������ѧ����ʽΪ��2H2O2![]() H2O+O2����
H2O+O2����
��2������ƽ��������Ӧ�����������룬���������C��
������һ����������������Һ����ȷ���������Ǽ��㡢�������ܽ⣬���Բ�����ȷ��˳����ECADB��
����Һ���о�һ�ԣ������ƺõ���Һװ���Լ�ƿʱ�����������˼��Σ�����Ӱ����Һ��������������ʹ��Һ�����ʵ������������䣻
��3���ڹ��˹����У������ֽ����Һ�������ֽ��Ե������ʹ��Һ���еIJ��������������ձ����Ӷ�ʹ����Һ���ǣ��н���Һ���ձ����ɾ�ʱҲʹ����Һ���ǣ����Ի��ǵ�ԭ���У�����ֽ���𣬢��������ɾ�����©����Һ�泬����ֽ��Ե��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ����һ����ɫ���壬���ܺ���NaOH��Na2CO3��Na2SO4 ��NaCl�� Ba(NO3)2�е�һ�ֻ��֣�Ϊ̽������ɣ�ijѧϰС����Ʒ���������������ʵ��:
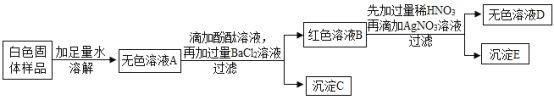
(1)����ʵ���У����˲����õ��IJ���������©�����ձ���______________��
(2)С��ͬѧͨ������ʵ����֪:��ɫ������Ʒ��һ��������______________��
(3)Ϊȷ����ɫ������Ʒ�п��ܴ��ڵ����ʣ�Сǿ�Գ���C����ʵ�顣
ʵ����� | ���� | ���ۼ���ѧ����ʽ |
ȡ��������C���Թ��У���������ϡ���� | �����ݲ���������������ʧ | ��ɫ������Ʒ��һ������__________�� �÷�Ӧ�Ļ�ѧ����ʽΪ:___________�� |
(4)С��ͬѧ��Ϊ��ɫ�����л������ʲ���ȷ�����Ƿ���ڣ���������:______________����Ҫȷ�ϣ�ֻ�轫����ʵ�鷽�������ӵ�һ���Լ���Ϊ______________���ɡ�
����Ŀ����ҵ���õ�ⱥ��ʳ��ˮ�ķ��������ռ�����������Ȼ�����Ʒ���䷴Ӧԭ��Ϊ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����ij�о���С����Ƶõ��ռ���Ʒ����������̽����
2NaOH+H2��+Cl2����ij�о���С����Ƶõ��ռ���Ʒ����������̽����
��������⣩�ռ���Ʒ�п��ܺ�����Щ����?
�����룩����һ����Ʒ¶���ڿ����е�����Ʒ���ܺ���̼���ơ�
�������ԭ��δ��ȫת��������Ʒ���ܺ����Ȼ��ơ�
��ʵ��̽��������ͬѧ�������̽��ʵ�飺
���� | ʵ�鲽�� | ʵ������ | ʵ����� |
����һ | ��ȡ�����ռ���Ʒ���Թ��У���ˮ�ܽ⣬�����еμӹ���ϡ���� | �����ݲ��� | __________ |
�ڽ�������в���������ͨ������ʯ��ˮ�� | ����ʯ��ˮ����� | ||
����� | ����������õ���Һ�еμӼ���AgNO3��Һ | ________ | ��Ʒ�к����Ȼ��� |
����������ۣ���ͬѧΪ����֤����һ��ʵ����ۣ�Ҳ���ռ���Ʒ����Һ�еμ�������ϡ���ᣬȴ���������ݲ�������Ҿ���������������ͬѧ�����Լ����ͬѧ��ͬ��ȴû�п������ݡ�ԭ�������_________��
�������뷴˼����ͬѧ��������ڼ�ͬѧ����ʵ���У�ϡ����Ҳ������ϡ������档������Ϊ���۵�_________(������ȷ����������ȷ��)��