��Ŀ����
����Ŀ����ѧ��ȤС���У��ס��ҡ�����λͬѧ��ij����������ˮȡ������������ʵ�飬��ش��������⡣
I.��pH��ֽ�����ˮ������ԡ���λͬѧ�ֱ��������ʵ��:
��ͬѧ:ȡpH��ֽ�ڱ�������,�ò�����պȡ����Һ����pH��ֽ�ϣ����pH<7.
��ͬѧ:ȡpH��ֽ�ڱ������ϣ���������ˮʪ�����ò�����պȡ����Һ����pH��ֽ�ϣ����pH<7��
��ͬѧ:ȡpH��ֱֽ�ӽ������Һ�У����pH <7.
(1)������λͬѧ�в����淶����___________ͬѧ��
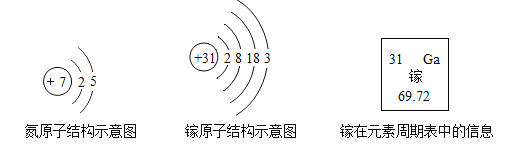
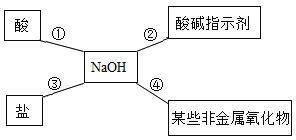
��.�����кͷ�Ӧԭ���ⶨ��ˮ����Ⱦ��(��Ϊ����)�������������ס�����ͬѧ��Ƶķ����ֱ���ͼ�ס�ͼ����ʾ:
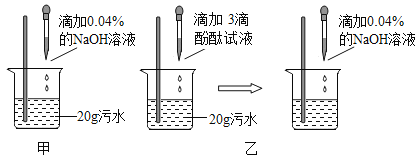
(2)��ͬѧȡ����ͼ����ʾ��Ӧ�����Һ���Թ��У��μ�3����ɫʯ����Һ����Һ����ɫ�����ǵó��ˡ�����������ǡ���к͡��Ľ��ۣ���ͬѧ�Ľ����Ƿ���ȷ?_____________ (ѡ���ȷ������ȷ��)��������______________��
(3)��ͬѧ��ͼ�ҷ�������ʵ�飬ʵ������У�NaOH��Һ�����ý�ͷ�ι���μ��룬���ò��������Ͻ��衣���۲쵽____________����ʱ�� ֹͣ�μ�NaOH��Һ����ʱ����Ϊ���ǡ����ȫ��Ӧ��
(4)��Ҫ��ȥ��ˮ�е����ᣬ�ӻ�����ԭ���ϳɱ��ȽǶȿ��ǣ����ѡ��________________��
A NaOH
B Ca(OH)2
C Fe2O3
D CaCO3
(5)��ͼ�ҷ���ʵ���й�������NaOH��Һ20g,�����:
��20g NaOH��Һ����������=______________g.
����ˮ��HCl�����������Ƕ���? ____ (��ʽ����)
���𰸡��� ����ȷ ��Һ����ɫ��˵������������Һ�ѹ��� ��ɫ��Һ�ոձ�Ϊ��ɫʱ D 0.008 0.0365%
��������
��1����pH��ֽ�ⶨpHֵ�ķ������ýྻ������IJ�����պȡ����Һ������pH��ֽ�ϣ��۲���ɫ�ı仯��Ȼ�������ɫ�����գ�����ֱ�ӽ�pH��ֽ�������Һ�У�����Ⱦ����Һ��Ӱ��ⶨ�����Ҳ���ܽ�pH��ֽ��ʪ����Ӱ��ⶨ������ʲ����淶���ǣ���ͬѧ��
��2���μ���ɫʯ����Һ����Һ����ɫ����ɫʯ����Һ���������˵������������Һ�������ʼ�ͬѧ�Ľ��۲���ȷ�������ǣ���Һ����ɫ��˵������������Һ�ѹ�����
��3����ɫ��̪��Һ�����Ժ�������Һ�в���ɫ���ڼ�����Һ��Ϊ��ɫ��������ˮ�еμ�����������Һ���������������ᷴӦ�����Ȼ��ƺ�ˮ������ɫ��Һǡ�ñ�Ϊ��ɫʱ��˵�����ǡ����ȫ��Ӧ�������ɫ��Һ�ոձ�Ϊ��ɫʱ��
��4���������ơ��������ơ�������������̼��ƾ��������ᷴӦ������̼��Ƽ۸���ͣ����Ҳ����������ơ���������һ�����и�ʴ�ԣ�̼��Ʋ�����ˮ�������̼��Ʋ�����Ⱦ��������ѡ��̼����������
��ѡD��
��5����20g NaOH��Һ����������Ϊ��20g��0.04%=0.008g��
�ڽ⣺����ˮ��HCl������������x
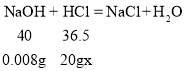
![]() x=0.0365%
x=0.0365%
����ˮ��HCl������������0.0365%��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ��Na2CO3��NaHCO3�����ƵĻ�ѧ���ʣ��������ᡢijЩ�Ӧ����̼�����ȶ����� NaHCO3���ȷֽ⣺2NaHCO3![]() X+H2O+CO2����
X+H2O+CO2����
��1��������X�Ļ�ѧʽΪ______��
��2����ҵ����̼�������ռ�Ļ�ѧ��Ӧ����ʽΪ______��
��3��ͬѧ����̽���仯ѧ����ʵ���ʣ�����ҩƷ���з����ձ��С�С��Ϊ��Ū��ʣ�����ijɷݣ����������µ�̽����
��������룩ʣ������ǣ�I��Na2CO3����NaHCO3����______��
������ʵ�飩
���� | ���� | ���� |
��ȡ����ʣ��������Թ��У���������ˮ������ܽ�μӼ��η�̪��Һ�� | ��Һ����ɫ���ɫ�� | ʣ�����һ���У� ______ �� |
��ȡ����ʣ��������Թ��У��̶�������̨�ϣ���ּ��ȡ� | ��ɫ�����������٣� �Թ��ڱ��н϶�ˮ����֡� |
�����۷�����
�پ�����ʵ������С����Ϊ�������ȷ������ͬѧ��ΪС�����жϲ��Ͻ�����Ϊ����______Ҳ����ͬ����
��ͬѧ�����ۺ���Ϊ����Ҫȷ��ʣ�����ɷ֣�С��ֻҪ��ʵ������ڣ����������______����ͨ��______���ɵó����ۡ�
����չ��˼��
��ͬѧ�����̼��ƺ�̼������ܲ��ܻ���ת���أ�С����Ϊ���ԣ���α������Ͽ�Ƭ���н��ܵġ�ʯ�������ʯ���γɡ�������CaCO3��Ca(HCO3)2��CaCO3���Ĺ��̡�����CaCO3��Ca(HCO3)2����ѧ��Ӧ����ʽΪ______��
����Ŀ��ij��ʯ��Ҫ�ɷ��� MgO���������� Fe2O3��CuO �� SiO2 ���ʡ��øÿ�ʯ�Ʊ� Mg(OH)2 �Ĺ������̼�ͼ��ͼ��

�ش��������⣺
��1������ٺͲ���ھ��õ��IJ�����_____________��
��2��������мӹ���ϡ�����Ŀ����______________��
��3����֪���ֽ��������������������γɳ���ʱ��Һ�� pH���±���
��Ӧ���� | Fe3+ | Cu2+ | Mg2+ |
��ʼ����ʱ�� pH | 1.9 | 4.2 | 9.1 |
��ȫ����ʱ�� pH | 3.2 | 6.7 | 11.1 |
����ڼ�����ʯ�ң�������Һ�� pH ��ΧΪ________________��