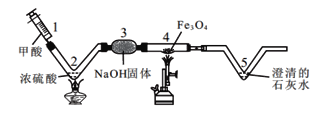
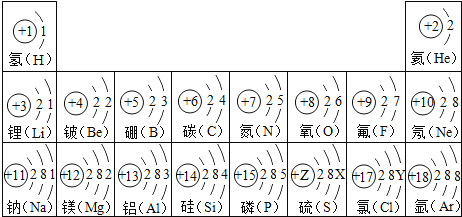
��Ŀ����
����Ŀ�������ǵ��������ľ۱��裬��嫵ĺ����̺��ŷḻ�Ļ�ѧ��Դ����ˮ�л�ѧ��Դ�����þ��зdz�������ǰ����Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ��Ӻ�ˮ����ȡþ����Ҫ������ͼ1��
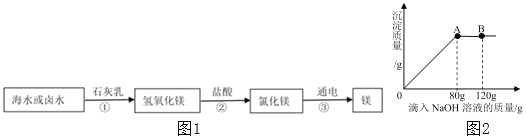
��1��������ˮ��þ�Ĺ��������ڷֽⷴӦ����______���������ĸ��
��2��д�����̢ڷ�����Ӧ�Ļ�ѧ����ʽ______��
��3��ij��ȤС���ͬѧȡ100gMgCl2��Һ����������μ���10%��NaOH��Һ���������������������NaOH��Һ��������ϵ��ͼ2��ʾ��������120gNaOH��Һ����ͼ��B�㣩ʱ����Һ�е�������______���ѧʽ����
��4��������80gNaOH��Һ����ͼ��A�㣩ʱ��ǡ����ȫ����Ӧ��ͨ�����㣬���ʱ���ò�������Һ������______����������ȷ��0.1g��
���𰸡��� ![]() NaCl��NaOH 174.2g
NaCl��NaOH 174.2g
��������
��1���ɺ�ˮ��ȡ�Ĺ��̼������ķ�Ӧ��֪�����Т۷ֽ��Ȼ�þ�õ���þ�����������ڷֽⷴӦ��
��2�����̢ڷ����ķ�Ӧ��������þ��ϡ�����������Ȼ�þ��ˮ����ѧ����ʽΪ��![]() ��
��
��3����ͼ���֪�����뵽80g������������Һʱ���Ȼ�þ����������ǡ�÷�Ӧ���������Ȼ��ƺ�������þ���������뵽120gʱ����ʣ����������ƣ�������Һ�к��е������ǣ�NaCl��NaOH��
��4�������ɵ�������þ������Ϊx��
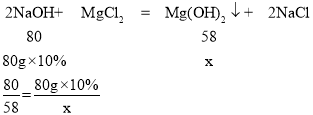
��ã�x=5.8g
��ʱ���ò�������Һ�������ǣ�100g+80g-5.8g=174.2g