��Ŀ����
տ����ˮ�೧�����ң�Ϊ�˲ⶨij��ɽʯ��ʯ��̼��Ƶ�����������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦҲ������ˮ�����й�ʵ���������±���
��1�����������غ㶨�ɿ�֪����Ӧ�����ɶ�����̼������Ϊ g��(2��)
��2�����ʯ��ʯ��̼��Ƶ�����������������д����ϸ������̣����������0.1%��(5��)
| | ��Ӧǰ | ��Ӧ�� | |
| ʵ �� �� �� | �ձ���ϡ���� ������ | ʯ��ʯ��Ʒ ������ | �ձ������л� ��������� |
| 150g | 12g | 157.6g | |
��2�����ʯ��ʯ��̼��Ƶ�����������������д����ϸ������̣����������0.1%��(5��)
��1��4.4
��2��83.3%
��1�� 4.4 ��2�֣���
��2����5�֣��⣺̼��Ƶ�����ΪX
CaCO3+ 2HCl CaCl2��H2O+CO2����������������������������1��
100 44
X 4.4g��������������������������1��
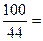
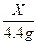 ��������������������������������1��
��������������������������������1��
��ã�X="10g " ������������������������������1��
ʯ��ʯ��̼��Ƶ���������Ϊ��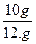 ��100% = 83.3%������1��
��100% = 83.3%������1��
��ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��
��2����5�֣��⣺̼��Ƶ�����ΪX
CaCO3+ 2HCl CaCl2��H2O+CO2����������������������������1��
100 44
X 4.4g��������������������������1��
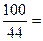
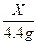 ��������������������������������1��
��������������������������������1����ã�X="10g " ������������������������������1��
ʯ��ʯ��̼��Ƶ���������Ϊ��
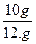 ��100% = 83.3%������1��
��100% = 83.3%������1����ʯ��ʯ��̼��Ƶ���������Ϊ83.3%��

��ϰ��ϵ�д�
�����Ŀ
 ��������һλС����
��������һλС����